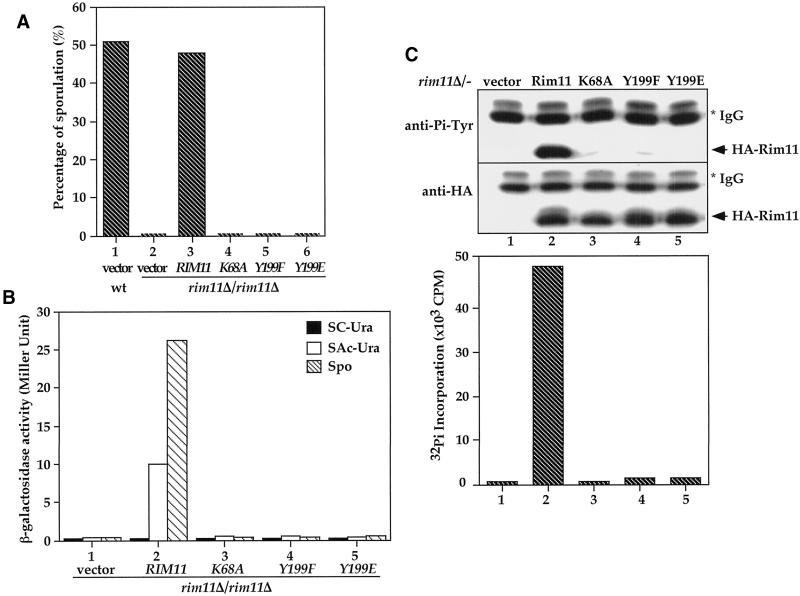
Figure 7.
Tyrosine phosphorylation of Rim11 at the Tyr-199 residue is required for Rim11 kinase activity and its in vivo function. (A) Tyrosine phosphorylation and kinase activity are essential for RIM11 function in sporulation. Wild-type (KB600, column 1) cells harboring control vector or rim11Δ/rim11Δ deletion cells (KB268) containing vector (pRS426, column 2), HA-tagged wild-type RIM11 (pKB166, column 3), rim11K68A (pKB199, column 4), rim11Y199F mutant (pKB201, column 5), or rim11Y199E (pXZ356, column 6) were cultured in sporulation medium. The percentage of sporulated cells after 24 h was determined by microscopic counting. (B) Tyrosine phosphorylation and kinase activity are essential for Rim11 function in ime2-lacZ induction. An a/α rim11Δ/rim11Δ ime2-lacZ/IME2 diploid was transformed with vector (column 1), HA-tagged Rim11 wild-type (pKB166, column 2), K68A (pKB199, column 3), Y199F (pKB201, column 4), or Y199E mutant (pXZ356, column 5) and cultured in glucose and acetate or 8 h after cells were shifted to sporulation media. The expressions of ime2-lacZ in glucose (SC-Ura, closed bar), acetate (SAc-Ura, open bar), and sporulation medium (Spo, hatched bar) were determined by β-galactosidase assay. Activities are in Miller units and are averages of two duplicated assays. Y199F and Y199E mutations eliminate Rim11 in vivo kinase activity. HA-tagged Rim11 (pKB166), Rim11K68A (pKB199), Rim11Y199F (pKB201),or Rim11Y199E (pXZ356) was expressed in rim11Δ/rim11Δ deletion cells (KB268). The HA-tagged proteins were immunoprecipitated from yeast cell lysates with anti-HA antibody. The Tyr phosphorylation (top), protein levels (middle), and kinase activity (bottom) were determined by anti-Pi-Tyr, anti-HA immunoblotting, or in vitro kinase assay.