Figure 4.
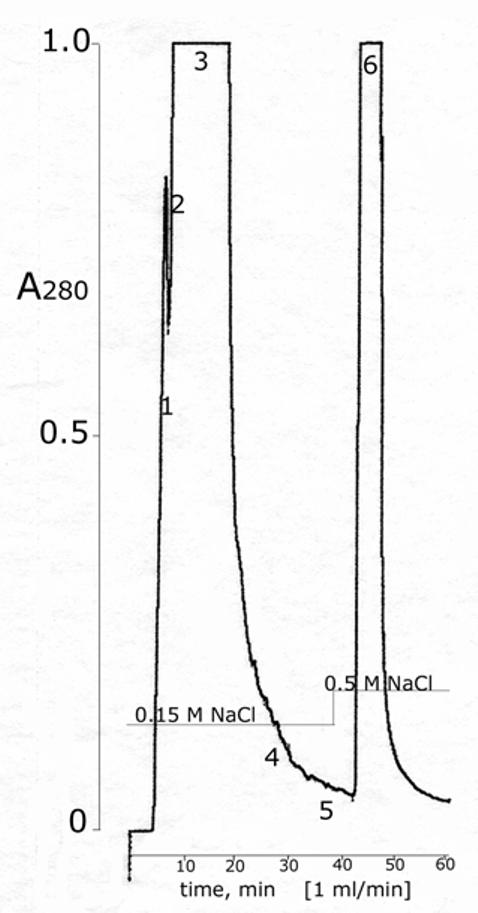
HPLC purification of BChE The 64% pure BChE recovered from the affinity column was desalted to about 0.04 M NaCl, reduced in volume, and filtered into a 25 ml sample loop in preparation for loading onto the Protein-Pak DEAE column. About 600 ml of 11 mM potassium phosphate pH 7.4 were pumped through the sample loop to load all of the BChE onto the HPLC column. The BChE was eluted with 0.15 M NaCl in 11 mM potassium phosphate pH 7.4 at a flow rate of 1 ml per min, at ambient temperature, at 400 psi. Fractions 1 and 2 at the leading edge of the peak, between 4.6-7.0 min, were 25% and 34% pure and contained 180 units out of the 18,200 units applied. Fraction 3 between 7.0-18.5 min was 80% pure and contained 16,100 units. Fractions 4 and 5 at the tail end of the peak between 18.5 and 38 min were 74% and 43% pure and contained a total of 970 units. Fraction 6, which contained additional contaminating proteins as well as 190 units of 5% pure BChE, eluted with 0.5 M NaCl.
