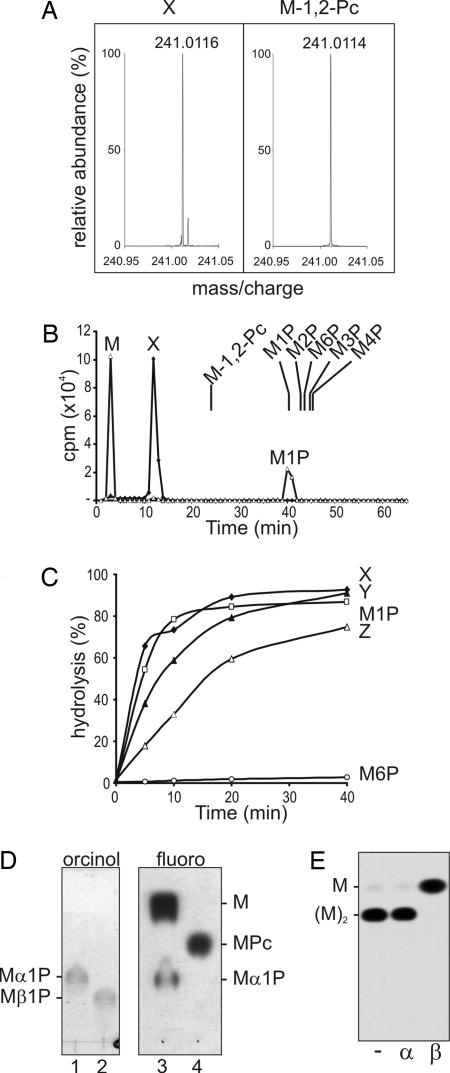
Fig. 2.
Characterization of the Man-α1,4-cyclic P primer. (A) Fourier transform ion-cyclotron resonance MS of authentic Man-β-1,2-cyclic P and peak X. (B) Mild acid hydrolysis of peak X releases Man-1-P. HPAEC analysis of peak X before (diamonds) and after (triangles) mild acid hydrolysis (50 mM TFA for 10 min at 100°C) under conditions that converted 50% of starting material to free Man (see C). The elution positions of authentic Man phosphates, Man-1-P, Man-2-P, Man-3-P, Man-4-P, Man-6-P, and Man-β-1,2-cyclic P, synthesized herein are indicated. (C) Kinetics of release of Man or mannan oligomers from peaks X–Z after mild acid hydrolysis. Peak X displayed the same acid sensitivity as Man-α-1-P, whereas Man-6-P was resistant to mild acid hydrolysis. (D) Peak X contains an α-linked mannosyl residue. Shown is HPTLC analysis of authentic Man-α-1-P (lane 1) and Man-β-1-P (lane 2) and Peak X after (lane 3) and before (lane 4) mild acid hydrolysis as described in B. Lanes 1 and 2 were stained with orcinol/H2SO4 (orcinol); lanes 3 and 4 were exposed to film (fluoro). (E) Peak Y contains terminal β-Man. Peak Y was acid-dephosphorylated and digested with mock control (−), jack bean α-mannosidase (α), or snail β-mannosidase (β). The products were analyzed by HPTLC and detected by fluorography.