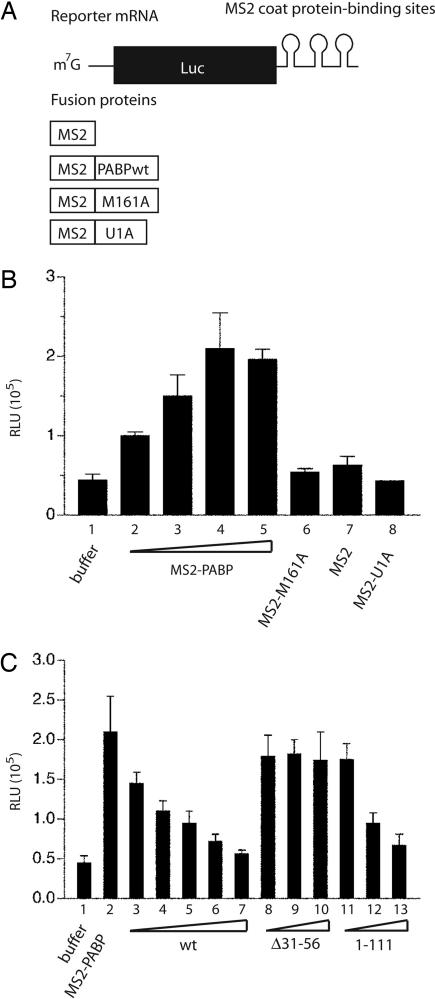
Fig. 5.
Paip2 negates the stimulatory activity of tethered PABP. (A) Diagram of the components used for the assay of the tethered PABP function. The capped reporter luciferase mRNA (Luc-MS2) was not polyadenylated but contained binding sites for MS2 coat protein within its 3′ UTR. The fusion proteins contained the MS2 coat protein sequence at their N terminus. (B) Tethered functional analysis of PABP. The assay was conducted in a PABP-depleted Krebs-2 cell extract. Translation reactions (10 μl) containing increasing concentrations of MS2–PABP (2.8, 5.5, 8.2, and 11 μg/ml in lanes 2, 3, 4, and 5, respectively) were incubated at 30°C for 1 h with 25 ng of Luc-MS2 mRNA. Translation was measured by luciferase assay and is expressed in relative light units (RLU). As negative controls, MS2 protein, either alone or fused to U1A, or PABP mutant M161A were used (at 82 μg/ml). (C) Inhibition of the tethered PABP function by Paip2. Luc–MS2 mRNA translation was assayed as in B in the presence of 82 ng (1 pmol) of MS2–PABP and increasing amounts of recombinant Paip2 WT or its mutants, Δ31–56 or 1–111 [used at the MS2–PABP to Paip2 molar ratios of 1:1 (lanes 3, 8, and 11), 1:2 (lanes 4, 9, and 12), 1:4 (lanes 5, 10, and 13), 1:8 (lane 6), or 1:16 (lane 7)]. In B and C the levels of luciferase in the presence of buffer (25 mM Hepes-KOH, pH 7.3/50 mM KCl/1.5 mM MgCl2/1 mM DTT) are shown in lane 1. The data are the average from three experiments with standard deviations from the mean.