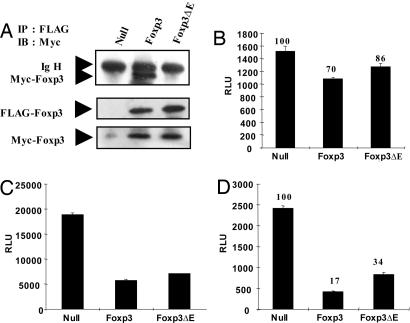
Fig. 6.
The glutamic acid residue in the leucine-zipper domain of Foxp3 is required for homodimerization. (A) FLAG- and Myc-tagged Foxp3 or Foxp3ΔE were cotransfected for 48 h. After harvesting of cells, lysates were incubated with FLAG antibody (M2)-conjugated agarose beads, washed and boiled with sample buffer for SDS/PAGE. Immunoprecipitates were analyzed by anti-Myc Ab. Lysates were also analyzed for protein expression by using anti-FLAG and anti-Myc Abs. RLU, relative luciferase unit. (B–D) Each null vector, Foxp3, and Foxp3 ΔE was cotransfected with the FKH luciferase construct in Jurkat T cells with lipid-mediated transfection (B). After 48 h, luciferase activity was measured (C and D). Each NFAT and NF-κB luciferase construct was cotransfected with null vector, Foxp3, or Foxp3ΔE in HEK293 cells. After 48-h transfection, cells were harvested and assessed for luciferase activity. All samples were normalized by cotransfection and analysis of TK-Renilla activity. A representative result from at least three independent experiments is shown.