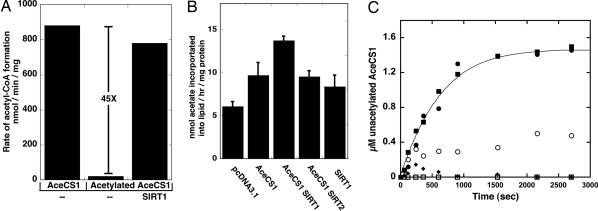
Fig. 2.
AceCS1 activity is regulated by sirtuin-catalyzed deacetylation. (A) AceCS1 activity is regulated by reversible acetylation. AceCS1 or acetylated AceCS1 (5 μM) was incubated for 1 h with 2 μM SIRT1 at 37°C. AceCS1 activity was determined by measuring the formation of acetyl-CoA over time from [14C]acetate, and reported as nmol of acetyl-CoA per min per mg of protein. (B) 1,2-[14C]acetate incorporation into lipids through AceCS1 is enhanced upon SIRT1 coexpression. Cos-7 cells were cotransfected with a vector encoding AceCS1 and/or SIRT1, SIRT2, or empty vector where indicated. Lipids were extracted as described in Materials and Methods, and [14C]acetate incorporation was analyzed by scintillation counting and normalized to protein content. ANOVA statistical analysis was performed, indicating significant differences (P < 0.05) between SIRT1 and AceCS1 coexpression and AceCS1 expression alone. (C) SIRT1 and SIRT3 show high catalytic activity against acetylated AceCS1. Catalytic amounts of SIRT1 (filled circles), SIRT2 (open circles), SIRT3 (filled squares), SIRT5 (filled diamonds), and SIRT6 (open squares) (50 nM) were incubated with 1.5 μM acetylated AceCS1 in 1 mM NAD+, 1 mM DTT, and 50 mM Tris (pH 7.5) at 37°C for the indicated time and deacetylation of AceCS1 was measured by AceCS1 activity assays. Data from SIRT1 and SIRT3 were fitted to the integrated Michaelis–Menten equation (45).