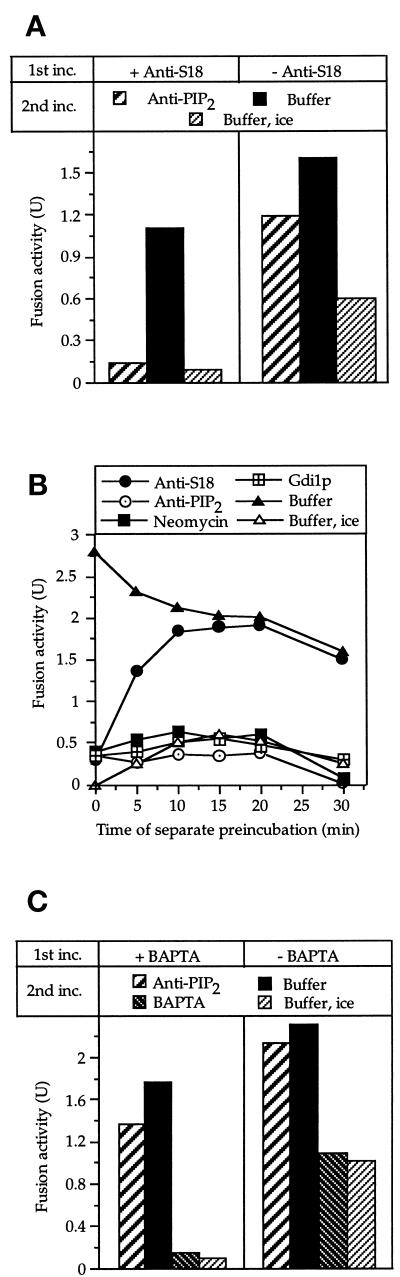
Figure 6.
Absolute order of steps. (A) Sec18p action (priming) must precede the PI(4,5)P2-dependent step. Standard fusion reactions were started in the presence or absence of 0.5 μM affinity-purified antibody to Sec18p. After 20 min at 27°C, the samples received antibodies to PI(4,5)P2 (70 μM) or buffer. After 5 min at 27°C, dithiothreitol (75 μM) and Sec18p (0.2 μM) were added to reactivate the Sec18p pathway. A control sample received only buffer. After further incubation for 70 min at 27°C or on ice, fusion was measured. (B) The requirement for PI(4,5)P2 cannot be satisfied before docking. Two separate samples, each equivalent to six standard fusion reactions, were incubated at 27°C. One contained BJ3505 vacuoles carrying proalkaline phosphatase, whereas the other contained DKY6281 vacuoles harboring the maturation enzyme, proteinase A. At the indicated times, aliquots (30 μl) were withdrawn from the reactions and chilled. The aliquots received antibodies to Sec18p (1 μM), neomycin (500 μM), Gdi1p (3 μg), PI(4,5)P2 (70 μM), or buffer only and were left on ice for 10 min. The fusion partners were combined in one tube, centrifuged briefly (1 min, 8000 × g, 4°C), mixed by vortexing, and incubated for 70 min at 27°C or on ice. Then, fusion was assayed. (C) Relationship of the PI(4,5)P2 requirement to the BAPTA-sensitive step. Reaction mixtures of four times the standard volume were incubated (45 min, 27°C) in the presence or absence of 2 mM BAPTA. The samples were diluted 10-fold with 150 mM KCl, 10 mM PIPES/KOH, pH 6.8, and 200 mM sorbitol and centrifuged (8 min, 8000 × g, 4°C). The vacuoles were resuspended in fresh reaction buffer and split into four aliquots. Three aliquots received anti-PI(4,5)P2 (70 μM) or BAPTA (2 mM) or only buffer and were incubated further at 27°C. The fourth aliquot received buffer and was kept on ice to monitor the degree of fusion that had occurred during the first incubation. After 60 min, fusion was assayed.