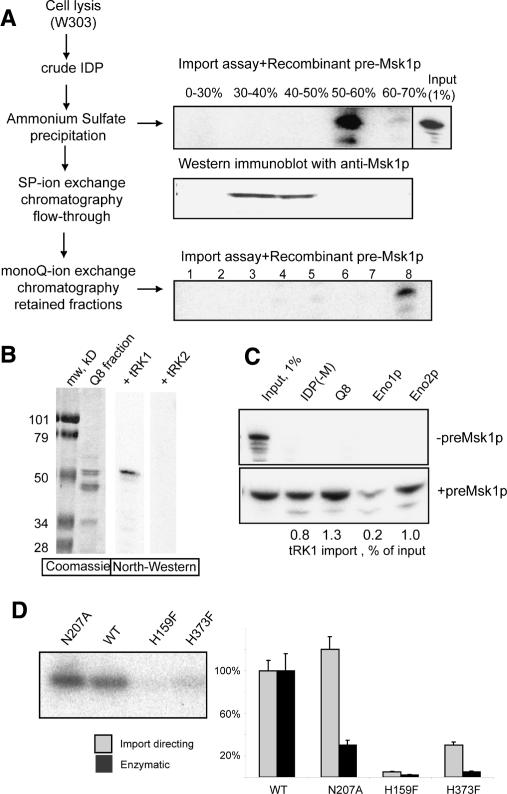
Figure 1.
Identification of import factors. (A) Strategy of proteins fractionation. (IDP) Protein extract. (Top and bottom right panels) In vitro import assays and autoradiographs of protected tRK1 after the incubation with isolated yeast mitochondria. (Input) An aliquot (1%) of labeled tRK1 used in each import assay. Ammonium sulphate saturation percentage and the numbers of mono-Q fractions are indicated. The middle panel represents results of Western immunodetection of preMsk1p in the same ammonium sulphate fractions as above. (B) North–Western analysis of the proteins from the mono-Q fraction 8 (Q8). The left panel is the Coomassie-stained gel, and the two panels on the right show autoradiographs after incubation of proteins with 5′-[32P]-labeled tRK1 or tRK2. (C) In vitro tRK1 import assay with fraction Q8 proteins and recombinant Eno1p or Eno2p in the presence or absence of preMsk1p. [IDP(−M)] IDPs lacking preMsk1p. tRK1 import efficiencies are indicated below the autoradiograph. (D) Comparison of enzymatic and tRK1 import-directing activities of a normal (wild-type [WT]) and mutant recombinant versions of Eno2, containing the following substitutions: N207A, H159F, or H373F. On the left is the in vitro import assay in the presence of recombinant preMsk1p, with Eno2p versions indicated above; on the right is the quantification of import-directing efficiency and enolase enzymatic activity of the recombinant proteins.