Abstract
To understand the mechanism of methamphetamine (MAP) craving from the viewpoint of nicotinic acetylcholinergic transmission, we examined the responsible site of the brain for anticraving effects produced by nicotinic agonists by using a MAP self-administration paradigm in rats. Systemic nicotine and an acetylcholinesterase inhibitor, donepezil, attenuated the reinstatement of MAP-seeking behavior by means of the activation of nicotinic acetylcholinergic receptors, but not muscarinic acetylcholinergic receptors, in the nucleus accumbens core, prelimbic cortex, amygdala, and hippocampus. Among these regions, with the exception of the hippocampus, we also found functional differences in this reinstatement. The nicotinic antagonist mecamylamine alone did not reinstate MAP-seeking behavior. These results suggest that the inactivation of nicotinic acetylcholinergic transmission may be an essential factor in the appearance of MAP-seeking behavior, and, thus, the normalization of the inactivated state may result in the suppression of the reinstatement. Our findings also indicate that there are functional differences in the responsible brain subregions. Extending this view to the treatment of MAP dependence, our results suggest that activators of nicotinic acetylcholinergic transmission are possible anticraving agents.
Keywords: craving, reinstatement, self-administration
The abuse of the powerfully addictive psychostimulant methamphetamine (MAP) is a growing problem worldwide (1). Many reports on MAP have focused on rewarding effects, drug-taking behavior, or hyperlocomotor activity, whereas few studies have examined the reinstatement of MAP-seeking behavior, a model of human craving.
Nicotine is generally known to cause dependence, suggesting the involvement of nicotinic acetylcholine receptors (nAChRs) in the reward system. Recently, the down-regulation of choline acetyltransferase and elevation of the expression of the vesicular acetylcholine transporter have been demonstrated in a MAP user’s brain (2), suggesting an inactivated state of acetylcholinergic transmission in the user’s brain. In addition, Hikida et al. (3) reported that donepezil, an acetylcholinesterase inhibitor, blocked cocaine-induced behavioral sensitization by means of acetylcholinergic activation in the nucleus accumbens. From this finding, it is hypothesized that the mechanism to induce MAP craving may involve an inactivated state of acetylcholinergic transmission; however, the roles of nAChRs in craving are unknown. In this study, we clarified the roles of nAChRs in MAP-seeking behavior by using an i.v. MAP self-administration paradigm in rats. Furthermore, assuming that systemic nicotine attenuates the reinstatement of MAP-seeking behavior, we examined the responsible brain regions for the nicotine-induced attenuating effect on reinstatement.
Results
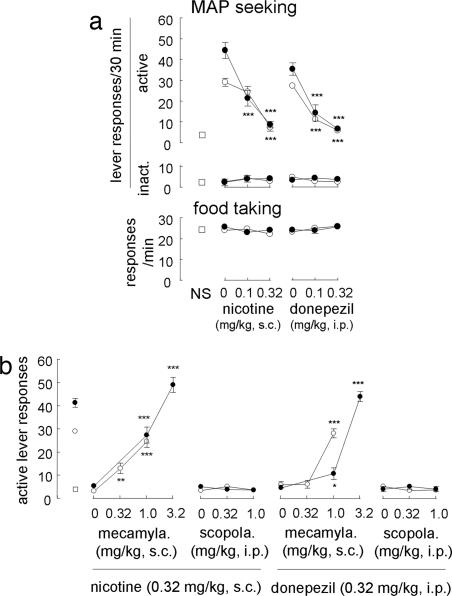
Rats were trained to self-administer MAP, followed by withdrawal sessions (on the final day of each phase, active lever responses were 35.8 ± 5.2 and 3.6 ± 0.7, respectively). Reexposure to the MAP-associated cue alone increased active lever responses in nicotine- and donepezil-challenged groups [F[1,15] = 119.0 and 198.4, respectively; P < 0.001 compared with the control (no pretreatments and nonstimuli)] (Fig. 1a). These increases were attenuated by nicotine and donepezil (F[3,27] = 42.7 and 39.1, respectively). In contrast, MAP priming alone also increased active lever responses in nicotine- and donepezil-challenged groups (F[1,12] = 112.6 and 108.5, respectively; P < 0.001 compared with nonstimuli). Similar to the cue-induced reinstatement, the MAP-primed increases in active lever responses were attenuated by nicotine and donepezil (F[3,24] = 43.2 and 31.9, respectively); however, these treatments did not alter inactive lever responses, a measure of nonspecific toxicity and food taking (P > 0.1 for both). Because we did not observe any significant effects of the following treatments (P > 0.1), data on inactive lever responses and food taking were omitted from all subsequent figures. However, mecamylamine, a nicotinic antagonist, blocked the effects of nicotine on reinstatement induced by a cue and MAP priming (F[2,21] = 29.7 and 60.4; P < 0.01 and 0.001 compared with the nicotine-pretreated group given the cue and MAP priming, respectively) (Fig. 1b). Scopolamine, a muscarinic antagonist, did not block the nicotine-induced attenuation of cue-induced and MAP-primed reinstatement (F[2,21] = 0.9 and 0.4; P > 0.4 compared with the cued and MAP-primed nicotine-pretreated group, respectively). In addition, mecamylamine blocked the effects of donepezil on reinstatement induced by a cue and MAP priming (F[2,21] = 59.9 and 114.8; P < 0.001 compared with the donepezil-pretreated group given the cue and MAP priming, respectively), whereas scopolamine did not antagonize the effect of donepezil on reinstatement induced by a cue and MAP priming (F[2,21] = 1.3 and 0.3; P > 0.3 compared with the cued and MAP-primed donepezil-pretreated group).
Fig. 1.
Involvement of nicotinic acetylcholinergic transmission in the reinstatement of MAP-seeking behavior (n = 7–16). (a) Effects of nicotine and donepezil on reinstatement (Upper) and food-taking behavior (Lower). Open squares, open circles, and filled circles represent groups given nonstimuli, MAP-associated cues, and MAP-priming injections, respectively. ∗∗∗, P < 0.001 compared with the cue and MAP priming alone. (b) Effects of the coadministration of mecamylamine and scopolamine with nicotine and donepezil on reinstatement (n = 7–16). ∗, ∗∗, and ∗∗∗, P < 0.05, 0.01, and 0.001, respectively, compared with nicotine- or donepezil-pretreated groups given the cue or MAP priming.
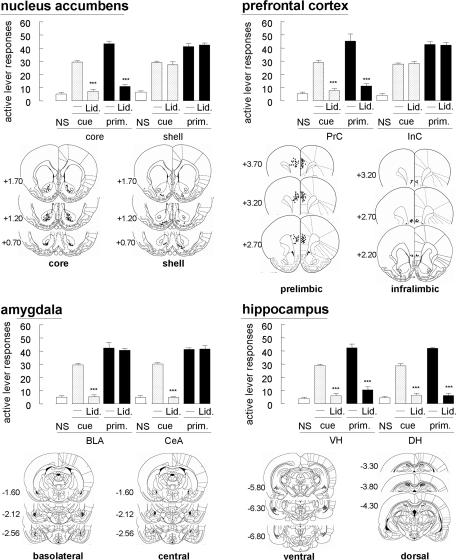
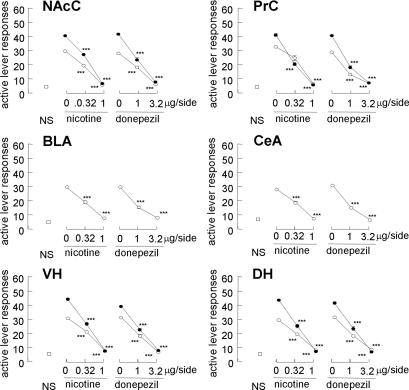
To identify the brain regions that are responsible for reinstatement, a local anesthetic, lidocaine, was used to induce reversible lesion. In the nucleus accumbens, intracranial lidocaine (100 μg per side) attenuated both the cue-induced and MAP-primed reinstatement when injected into the core [nucleus accumbens core (NAcC); F[1,12] = 143.9 and 180.7; P < 0.001, respectively] but not the shell [nucleus accumbens shell (NAcS); P > 0.1 for both] (Fig. 2). However, intracranial lidocaine into the prefrontal cortex attenuated both the cued and MAP-primed reinstatement when injected into the prelimbic cortex (PrC) (F[1,12] = 103.7 and 35.9; P < 0.001, respectively) but not the infralimbic cortex (InC) (P > 0.1 for both). Concerning the amygdala, intracranial lidocaine into the basolateral amygdala (BLA) and central amygdala (CeA) attenuated cue-induced reinstatement (F[1,12] = 154.2 and 389.6; P < 0.001, respectively); however, these treatments did not affect MAP-primed reinstatement (P > 0.1 for both). In addition, intracranial lidocaine into the ventral hippocampus (VH) and dorsal hippocampus (DH) attenuated cue-induced reinstatement (F[1,12] = 150.1 and 104.3; P < 0.001, respectively). Furthermore, these treatments attenuate MAP-primed reinstatement (F [1,12] = 71.9 and 125.3 in the VH and DH; P < 0.001, respectively). Intracranial nicotine and donepezil into NAcC attenuated the reinstatement induced by the cue (F[2,18] = 85.9 and 56.9; P < 0.001, respectively) (Fig. 3). These treatments also attenuated MAP-primed reinstatement (F[2,18] = 128.0 and 48.9; P < 0.001, respectively). In other regions, including the PrC, BLA, CeA, VH, and DH, cue-induced reinstatement was attenuated by intracranial nicotine (F [2, 18] = 19.8, 85.9, 85.9, 61.1, and 93.5; P < 0.001, respectively) and donepezil (F[2,18] = 48.0, 56.9, 56.9, 55.1, and 56.9; P < 0.001, respectively). Furthermore, MAP-primed reinstatement was blocked by intracranial nicotine into the PrC, VH, and DH (F[2,18] = 67.1, 56.3, and 128.0; P < 0.001, respectively) and donepezil (F[2,18] = 55.6, 64.2, and 48.9; P < 0.001, respectively).
Fig. 2.
Effects of intracranial lidocaine (Lid.) (100 μg per side) on reinstatement (n = 7–14) and schematic representations of the locations of intracranial cannula tips in the coronal section (14). ∗∗∗, P < 0.001 compared with the cue presentation or MAP priming alone. On the site maps, the numbers indicate the distance from the bregma in the anteroposterior plane. Mapping also includes the location of the cannula tips used in all studies.
Fig. 3.
Effects of intracranial nicotine and donepezil injected into regions where intracranial lidocaine affected cue-induced and MAP-primed reinstatement (n = 7–14). Open squares, open circles, and filled circles represent groups given nonstimuli, MAP-associated cues, and MAP-priming injections, respectively. ∗∗∗, P < 0.001 compared with the cue presentation and MAP priming alone.
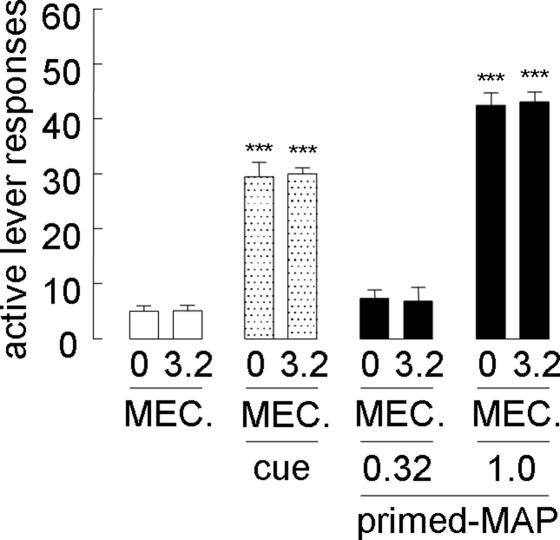
In tests with mecamylamine, intra-NAcC microinjection blocked the attenuating effect of systemic nicotine and donepezil on cue-induced reinstatement (F[2,18] = 18.6 and 40.4; P < 0.001 compared with the cued nicotine- and donepezil-pretreated groups, respectively) (Fig. 4). With regard to the MAP-primed reinstatement, intra-NAcC mecamylamine prevented the attenuating effect of systemic nicotine and donepezil (F[2,18] = 34.3 and 41.3; P < 0.001 compared with MAP-primed nicotine- and donepezil- pretreated groups, respectively); however, intra-NAcC scopolamine did not affect the nicotine- or donepezil-induced attenuating effect on both the cue-induced and MAP-primed reinstatement (P > 0.1 compared with cued or MAP-primed nicotine- and donepezil-pretreated groups). In other regions, including the PrC, BLA, CeA, VH, and DH, intracranial mecamylamine in cue-induced reinstatement attenuated the blocking effect of nicotine (F[2,18] = 32.8, 95.7, 30.5, 81.5, and 38.1; P < 0.001 compared with the cued nicotine-pretreated group, respectively) and donepezil (F[2,18] = 125.6, 71.9, 51.9, 62.6, and 55.1; P < 0.001 compared with the donepezil-pretreated group challenged with the cue, respectively). Furthermore, in the PrC, VH, and DH, intracranial mecamylamine blocked the attenuating effect of nicotine on MAP-primed reinstatement (F[2,18] = 51.2, 100.9, and 66.1; P < 0.001 compared with the MAP-primed nicotine-pretreated group, respectively) and donepezil (F[2,18] = 62.8, 77.2, and 72.8; P < 0.001 compared with the MAP-primed donepezil-pretreated group, respectively). However, intracranial scopolamine into these regions did not affect nicotine- or donepezil-induced attenuation of both the cue-induced and MAP-induced reinstatement (P > 0.1 compared with the cued or MAP-primed nicotine- and donepezil-pretreated groups).
Fig. 4.
Effects of intracranial mecamylamine and scopolamine injected into the subregions shown in Fig. 2 on the systemic nicotine- and donepezil-induced blocking effects on reinstatement (n = 7–14). Open squares, open circles, and filled circles represent groups given nonstimuli, MAP-associated cues, and MAP-priming injections, respectively. ∗∗∗, P < 0.001 compared with nicotine- or donepezil-pretreated groups given the cue and MAP priming.
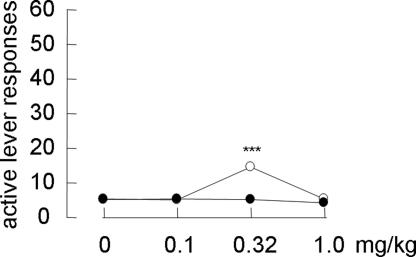
Systemic mecamylamine alone (3.2 mg/kg s.c.) neither reinstated MAP-seeking behavior (Fig. 5) nor affected cue-induced and MAP-primed reinstatement (Fig. 5). In addition, systemic mecamylamine (3.2 mg/kg s.c.) did not alter the response with MAP priming at 0.32 mg/kg i.p., which did not reinstate MAP-seeking behavior by itself (Fig. 5). In contrast, systemic nicotine priming (0.32 mg/kg s.c.), but not donepezil priming (0.1–1.0 mg/kg i.p.), reinstated MAP seeking in an inverted U-shaped manner (F[3,28] = 24.8; P < 0.001) (Fig. 6) but to a much lesser degree than cue-induced and MAP-primed reinstatement.
Fig. 5.
Effects of systemic mecamylamine alone on reinstatement (n = 8–16). Open, dotted, and filled bars represent groups given nonstimuli, MAP-associated cues, and MAP-priming injections, respectively. ∗∗∗, P < 0.001 compared with a group challenged with nonstimuli.
Fig. 6.
Effects of systemic nicotine and donepezil alone on the reinstatement of MAP-seeking behavior (n = 8). Open and filled circles represent groups pretreated with nicotine and donepezil, respectively. ∗∗∗, P < 0.001 compared with nonstimuli.
Discussion
Reexposure to MAP-associated cues and MAP priming reinstated MAP-seeking behavior, and systemic nicotine and donepezil attenuated this reinstatement. The attenuating effects of nicotine and donepezil were blocked by systemic mecamylamine, a nicotinic antagonist, but not by a muscarinic antagonist, scopolamine. These results suggest that the attenuating effects of nicotine and donepezil may occur by means of nicotinic acetylcholine receptors; they were not due to the nonspecific inhibition of lever-pressing behavior because of no alteration of food-reinforced lever-pressing behavior. Cue-induced reinstatement was attenuated by intracranial lidocaine, a local anesthetic, into the NAcC, PrC, BLA, CeA, VH, and DH but not the NAcS and the InC; however, MAP-primed reinstatement was also attenuated by intracranial lidocaine into the NAcC, PrC, VH, and DH. In brain regions where lidocaine attenuated MAP-seeking behavior, intracranial nicotine and donepezil also attenuated reinstatement. In addition, the attenuating effects of systemic nicotine and donepezil were reversed in each case by intracranial mecamylamine into these regions, but not by scopolamine. In contrast, systemic mecamylamine alone neither reinstated MAP-seeking behavior nor affected cue-induced and MAP-primed reinstatement. Furthermore, systemic mecamylamine with MAP priming at a dose that did not reinstate MAP-seeking behavior by itself did not affect lever responses. These results suggest that the inactivation of nicotinic acetylcholinergic transmission is an essential factor for the appearance of MAP-seeking behavior, and, thus, the normalization of the inactivated state may result in the suppression of the reinstatement. In a clinical study, it has been demonstrated that the down-regulation of choline acetyltransferase and elevation of the expression of the vesicular acetylcholine (ACh) transporter develop in a MAP user’s brain (2). This result suggests that elevation of the expression of the vesicular ACh transporter may be due to compensation for the down-regulation of choline acetyltransferase and that these changes may lead to the inactivated state of nicotinic acetylcholinergic transmission. Our results appear to be in line with this notion.
It is stressed in this study that the amygdala was involved in cue-induced but not MAP-primed reinstatement. In cocaine-seeking behavior, inactivation of the amygdala attenuates cocaine-associated cue-induced reinstatement (4–6). Therefore, the amygdala appears to be specifically involved in the effect of drug-associated cue. In addition, intracranial lidocaine injected in the NAcC and PrC, but not the NAcS and InC, attenuated reinstatement induced by both the cue and primed MAP. The results suggest that these two regions have functional differentiation in each subregion. Similarly, in MAP-seeking behavior, functional differentiation in the nucleus accumbens and prefrontal cortex subregions is reported in food-taking behavior (7) and behavioral cocaine sensitization (8). Anatomically, the InC projects preferentially to the NAcS (9) (where lidocaine showed no attenuation of MAP-seeking behavior), whereas the PrC, amygdala, and hippocampus, where lidocaine attenuated reinstatement, have close reciprocal neuronal projections and project preferentially to the NAcC (10). Furthermore, the PrC has a strong projection to the ventral tegmental area, reported as one of the brain regions that is responsible for the expression of cocaine-seeking behavior (11). Projection fibers from the nucleus accumbens reached the nucleus basalis of Meynert and formed synaptic contacts with cholinergic neurons, which project to diverse neocortical areas (10). In addition to the neocortex, the amygdala and hippocampus also exhibit cholinergic afferent projection (12).
We studied whether systemic nicotine and donepezil alone would reinstate MAP-seeking behavior. Although nicotine alone, but not donepezil, reinstated MAP-seeking behavior, it was marginal compared with cue-induced and MAP-primed reinstatement. Based on these results, donepezil would appear more useful than nicotine as an anticraving agent. A clinical study also demonstrated that nicotine produced milder subjective effects and weaker psychic withdrawal symptoms than MAP (13). These results suggest that not only donepezil but also nicotine preparations, such as nicotine patches, can be effective for the attenuation of MAP-seeking behavior.
In summary, the inactivation of nicotinic acetylcholinergic transmission may be an essential factor in the appearance of MAP-seeking behavior, and, thus, the normalization of the inactivated state may result in the suppression of the reinstatement. Furthermore, the craving mechanisms induced by a MAP-associated cue and MAP priming are not necessarily the same, with reference to the responsible regions in the brain. Extending the current view on the treatment of drug dependence, activators of the nicotinic acetylcholinergic transmission may be useful anticraving agents for MAP dependence.
Methods
Subjects and Histology.
Male Wistar/ST rats (250–350 g; Japan SLC, Hamamatsu, Japan) were surgically catheterized in the jugular vein and cannulated in various brain regions. After all experiments were completed, the locations of the intracranial cannula tips were verified as described in ref. 11. Intracranial guide cannulas were bilaterally implanted 1 mm above the NAcC (coordinates: anteroposterior, mediolateral, and dorsoventral, +1.2, ±1.6, and −7.8 mm relative to the bregma, midline, and skull surface, respectively), NAcS (+1.2, ±1.6, and −9.3), PrC (+3.2, ±0.75, and −4.7), InC (+2.7, ±0.75, and −5.9), BLA (−2.12, ±4.5, and −8.5), CeA (−2.12, ±3.6, and −8.4), VH (−6.3, ±4.8, and −7.9), and DH (−3.80, ±1.8, and −3.7). Procedures for animal treatments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the Declaration of Helsinki and Faculty of Pharmaceutical Sciences of Kyushu University.
Drugs.
MAP HCl (Dainippon Pharmaceutical, Osaka), (−)-nicotine di-(+)-tartrate (Research Biochemicals, Natick, MA), donepezil (a gift from Eisai, Tsukuba, Japan), mecamylamine (Sigma-Aldrich), (−)scopolamine (Sigma-Aldrich), and lidocaine HCl (Sigma-Aldrich) were dissolved in saline. MAP was administered i.v. for self-administration (0.02 mg/0.1 ml per infusion) and i.p. for priming injection (1.0 mg/kg) 30 min before tests. Nicotine and mecamylamine were administered s.c. 30 min before tests; donepezil and scopolamine were administered i.p. Drugs were microinjected into the brains 5 min before tests through injection cannulas (inner and outer diameters were 0.1 and 0.35 mm, respectively; a stainless-steel tube was used) that extended 1 mm below the guide cannulas (stainless steel) using a microsyringe (Hamilton).
Training in Lever Pressing for Food Reinforcement.
Lever pressing was initially accomplished by training rats to press a lever for food-pellet reinforcement (45 mg; Bioserv, Frenchtown, NJ) in operant chambers under an FR1 schedule of reinforcement (each lever pressing was reinforced) followed by surgical operation. Lever-pressing training ceased when rats could obtain 30 pellets within 180 s for three consecutive sessions.
MAP Self-Administration, Withdrawal, and Reinstatement.
Two days after surgery, rats were trained to self-administer MAP under the FR1 schedule in a 2-h daily session for 10 days (MAP taking). Each injection was accompanied by a light and tone (MAP-associated cue). During this time, inactive lever responses had no programmed consequences, but the number was recorded. After MAP self-administration, a withdrawal session (1 h), during which active lever responding resulted in saline infusion instead of MAP and the MAP-associated cue was not presented, was given daily for 5 days (or until the rats reached the withdrawal criterion of <10 responses per session for the previously active lever). Reinstatement tests under saline infusion were carried out for 30 min every 3 days under the FR1 schedule. In the cue-induced test, immediately after the onset of the session, rats were reexposed to the MAP-associated cue, and each active lever pressing resulted in the cue presentation. In the MAP-primed reinstatement test, MAP (1.0 mg/kg i.p.) was injected 30 min before the test. Each response during the test session resulted in saline infusion but not the MAP-associated cue.
Operant Task Performance for Food Pellets.
All subjects pressed a lever for food-pellet reinforcement under the FR1 schedule 5 min after the self-administration session. Each test ended when rats had delivered 30 pellets. The time limit was 1,200 s.
Data Analysis.
Data represent the mean ± SE number of lever responses. Results of lever responses were analyzed by ANOVA (a within-subjects design). One-way ANOVA was used to compare the means, and Bonferroni–Dunn tests were used for post hoc analysis. Differences were considered significant at P < 0.05.
Acknowledgments
We thank Dr. K. Takada (Teikyo University, Tokyo) for his valuable comments.
Abbreviations
- MAP
methamphetamine
- NAcC
nucleus accumbens core
- NAcS
nucleus accumbens shell
- PrC
prelimbic cortex
- InC
infralimbic cortex
- BLA
basolateral amygdala
- CeA
central amygdala
- VH
ventral hippocampus
- DH
dorsal hippocampus.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lineberry T. W., Bostwick J. M. Mayo Clin. Proc; 2006. pp. 77–84. [DOI] [PubMed] [Google Scholar]
- 2.Siegal D., Erickson J., Varoqui H., Ang L., Kalasinsky K. S., Peretti F. J., Aiken S. S., Wickham D. J., Kish S. J. Synapse. 2004;52:223–232. doi: 10.1002/syn.20020. [DOI] [PubMed] [Google Scholar]
- 3.Hikida T., Kitabatake Y., Pastan I., Nakanishi S. Proc. Natl. Acad. Sci. USA. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitelaw R. B., Markou A., Robbins T. W., Everitt B. J. Psychopharmacology. 1996;127:213–224. [PubMed] [Google Scholar]
- 5.Lu L., Hope B. T., Dempsey J., Liu S. Y., Bossert J. M., Shaham Y. Nat. Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 6.Kantak K. M., Black Y., Valencia E., Green-Jordan K., Eichenbaum H. B. J. Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkinson J. A., Olmstead M. C., Burns L. H., Robbins T. W., Everitt B. J. J. Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzschentke T. M., Schmidt W. J. Cereb. Cortex. 2000;10:488–498. doi: 10.1093/cercor/10.5.488. [DOI] [PubMed] [Google Scholar]
- 9.Laubach M. G., Woodward D. J. J. Comp. Neurol. 1995;360:49–58. doi: 10.1002/cne.903600105. [DOI] [PubMed] [Google Scholar]
- 10.Pennartz C. M., Groenewegen H. J., Lopes da Silva F. H. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 11.McFarland K., Kalivas P. W. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer M. K., Eiden L. E., Weihe E. Neuroscience. 1998;84:331–359. doi: 10.1016/s0306-4522(97)00516-2. [DOI] [PubMed] [Google Scholar]
- 13.Miyata H., Kono J., Ushijima S., Yanagita T., Miyasato K., Fukui K. Ann. N.Y. Acad. Sci. 2004;1025:481–488. doi: 10.1196/annals.1316.059. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]