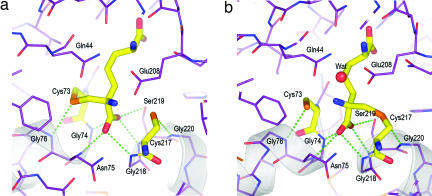
Fig. 3.
Hydrogen bonding at the catalytic center. (a) Close-up of the atomic details of the active-site DAP epimerase in the presence of the ll-AziDAP inhibitor covalently bound to Cys-73 Sγ. The α-carboxylate is the recipient of five hydrogen bonds (green dotted lines). Cys-73 Sγ and Cys-217 Sγ are both recipients of main-chain N···H hydrogen bonds from Gly-76 and Gly-220, respectively. Carbon atoms of the enzyme are shown in violet, nitrogen in blue, and oxygen in red. The carbon atoms of the inhibitor are shown in yellow, as are the carbon atoms of the catalytic cysteines. (b) Hydrogen-bonding interactions (green dotted lines) in the active site of DAP epimerase in the presence of the inhibitor dl-AziDAP. The atom coloring is the same as in a. dl-AziDAP is covalently bonded to Cys-217 Sγ.