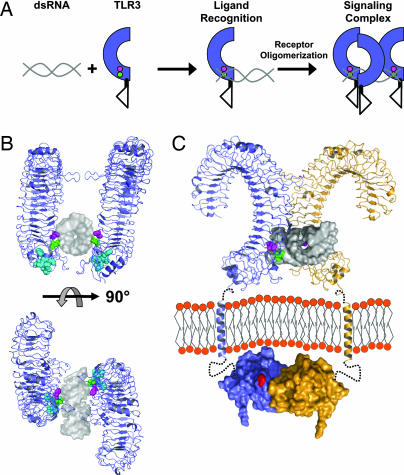
Fig. 6.
Model of TLR3 recognition of dsRNA and signaling complex. (A) Diagram of TLR3 ligand recognition and signaling complex. dsRNA is depicted in gray, and TLR3-ECD is shown in blue. H539 and N541 are shown as magenta and green circles, respectively. (B) A 19-bp dsRNA molecule (gray surface, Protein Data Bank ID code 1QC0) was docked onto the TLR3-ECD structure (blue). In the model, H539 (magenta) coordinates a phosphate group in the minor groove whereas N541 (green) forms a hydrogen bond to the 2′OH of the ribose also in the minor groove. The LRR20 insertion is shown in cyan. Two TLR3-ECD molecules, related by an ≈180° rotation, bind to one dsRNA ligand. Multiple TLR3 molecules could bind to a longer dsRNA as illustrated in A. (C) A proposed signaling complex of full-length TLR3 includes the oligomer model of ligand interaction from B rotated 45° with 22-residue transmembrane helices and TIR domains (Protein Data Bank ID code 1FYV). Single receptors are shown in blue or orange. Linker regions between the LRR-CT and TM (7 residues) and the TM and TIR domain (19 residues) are denoted by black dotted lines. The homologous position of the Lpsd mutation in the BB loop of the TLR4 TIR domain (25), postulated to interact with MyD88 (26), is indicated in red. Notably, the separation of the TLR3-ECD in the proposed oligomer easily accommodates the formation of a TIR dimer (27).