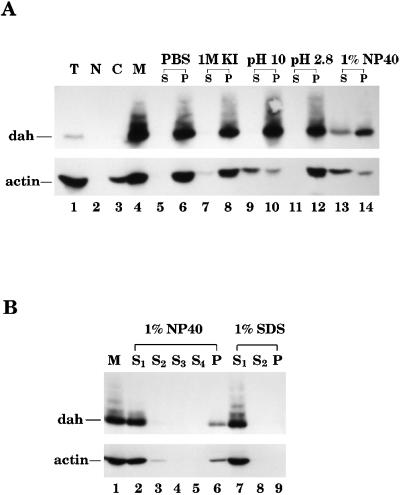
Figure 1.
DAH is tightly associated with membranes. (A) Embryos (0–4 h) were prepared for cellular fractionation on a sucrose step gradient. Total extract (T) and nuclear (N), cytosolic (C), and membrane (M) fractions were loaded on a SDS 8% polyacrylamide gel and subjected to Western blot analysis by DAH and actin antibodies. Twenty-five micrograms of total proteins was loaded in lanes 1, 2, and 4, and 12 μg was loaded in lane 3. DAH protein is concentrated in the membrane fraction (lane 4). Various solubilizing reagents were used to wash the membranes prepared from the fractionation experiment. These reagents were PBS, 1 M KI, 100 mM Na2CO3 (pH 10), 100 mM glycine (pH 2.8), and 1% NP-40. After washing, the same volume of supernatant (S) and pellet (P) was loaded on the gel and subjected to Western blot analysis. DAH protein can be solubilized only from the membrane by 1% NP-40 (lane 13). (B) The membrane fractions (M) underwent four washes in 1% NP-40 or two washes in 1% SDS. Equal volumes of the supernatants (S1, S2, S3, and S4) from each wash and the final pellet (P) were loaded onto a gel for Western blot analysis. A fraction of DAH remains insoluble in the nonionic detergent NP-40 (lane 6).