Figure 1.
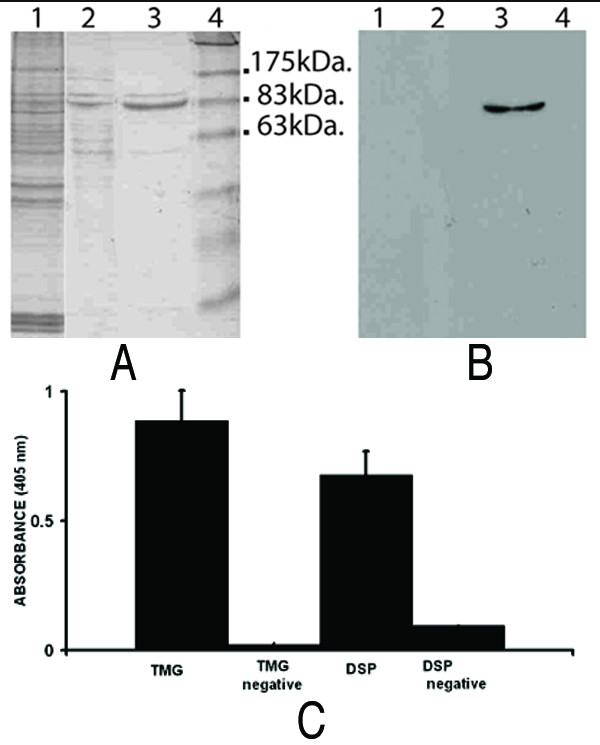
Purification of Hsp70 and Hsp70-PCs from MDA-MB-231 cells by affinity chromatography. Hsp70-peptide complexes (Hsp70-PCs) were isolated from whole cell extracts of MDA-MB-231 cells using ADP-Agarose. A. Coomassie-Blue stained SDS-polyacrylamide gel and B: Western blot using anti-Hsp70 antibody. Lane 1: MDA-MB-231 total cell extract (10 μg), Lane 2: Flow-through from an ADP-agarose column (2 μg), Lane 3: Proteins eluted from ADP-agarose column with 3 mM ADP (2 μg). Lane 4: Molecular weight markers. C: ELISA to detect the interaction between biotinylated TMG and DSP peptides and the corresponding phages. Streptavidin-coated paramagnetic beads bound to biotinylated TMG peptide (TMG) or DSP peptide (DSP) were incubated with the M13 phage clones displaying DSP or TMG respectively. As a control, streptavidin-coated beads without the peptides were incubated with M13 phage clone displaying the TMG (TMG negative) or the DSP (DSP negative) peptides alone. All beads were then incubated with anti-M13-HRP antibody. Interactions were detected by absorbance at 405 nm using DAB as a substrate.
