Figure 8. ABCA1 protein (A) and plasma membrane (B) levels in the presence or absence of Aramchol; effect of calpain (C).
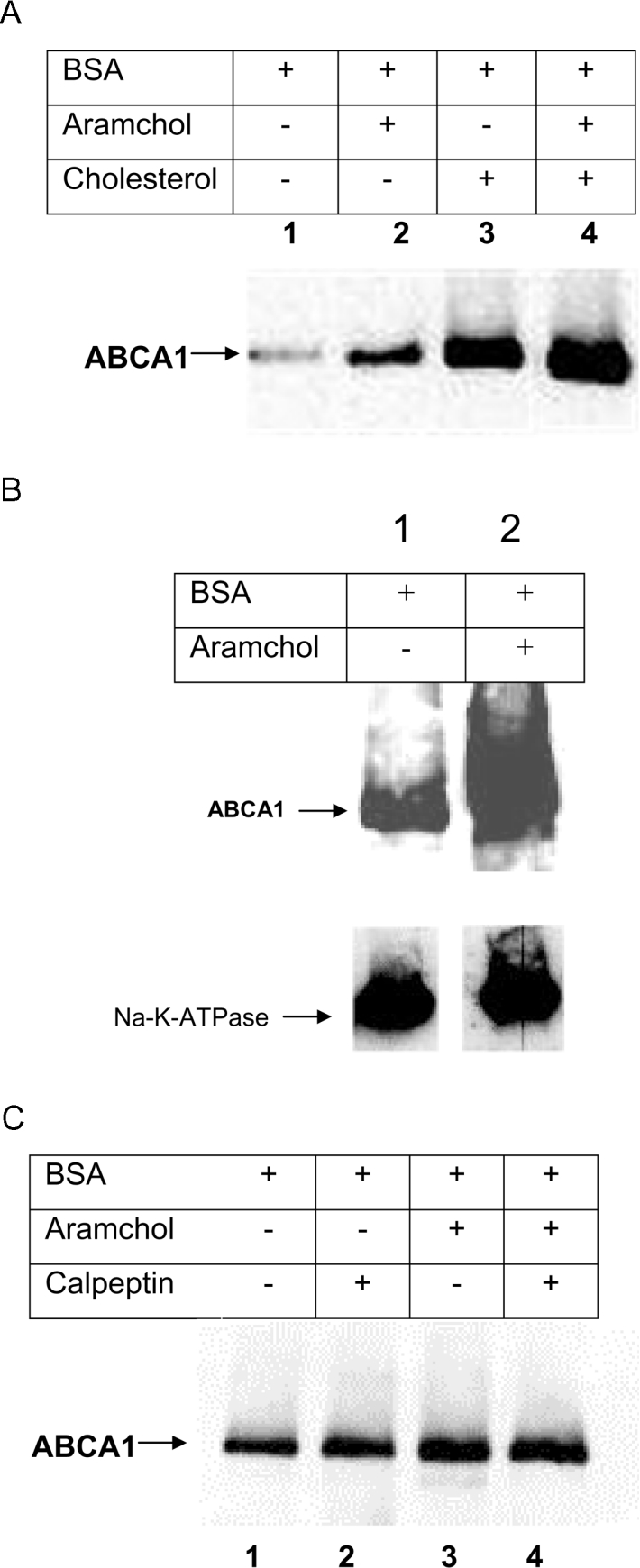
(A) Efflux from fibroblasts was measured in the presence, or absence of Aramchol (2 μg/ml). Subsequently, fibroblasts were harvested and protein extracts were subjected to Western blotting. Lane 1, no additions; lane 2, Aramchol (2 μg/ml); lane 3, cholesterol loading; lane 4, cholesterol loading plus 2 μg/ml Aramchol. (B) Cholesterol-loaded fibroblasts were incubated with, or without, Aramchol (2 μg/ml) and subjected to the efflux protocol. Subsequently the cells were put on ice and membrane proteins were biotinylated as described in the Materials and methods section. Biotinylated membrane fractions were isolated by streptavidin-affinity column chromatography. ABCA1 in these fractions was visualized by Western blotting. Lane 1, BSA; lane 2, BSA plus 2 μg/ml Aramchol. The blots were probed using anti-ABCA1 antibody, and after stripping with anti-Na+/K+-ATPase antibody to control for aspecific effects and equal loading of membrane protein. (C) Efflux from cholesterol-loaded fibroblasts was performed in the presence or absence of Aramchol (2 μg/ml) and with or without calpeptin (25 μM). After the incubations, cells were harvested and ABCA1 was visualized by Western blotting as described in the Materials and methods section. Lane 1, BSA; lane 2, BSA plus calpeptin; lane 3, BSA plus 2 μg/ml Aramchol; lane 4, BSA plus 2 μg/ml Aramchol plus calpeptin. Representative experiments are shown, and three independent experiments were carried out for all conditions.
