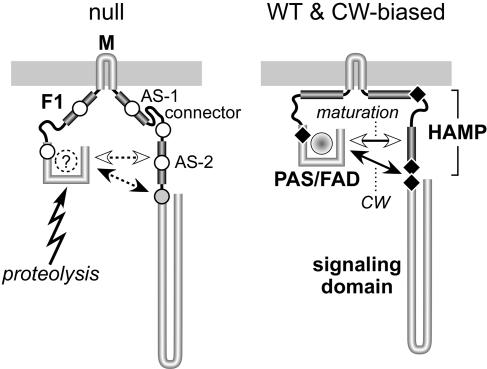
FIG. 9.
Possible structural basis for functional defects of null and CW-biased mutant Aer proteins. Although native Aer molecules most likely function as homodimers, only one subunit is shown. Some of the postulated interactions of Aer structural elements might take place between dimer subunits, rather than within one subunit, as illustrated. We propose that in native Aer molecules, the F1 and HAMP AS-1 segments contribute targeting and topology determinants that stabilize the membrane-associated conformation, thereby optimizing CW-inducing interactions between the PAS input domain and the AS-2/SD output region, which are modulated in response to redox changes in the PAS-associated FAD moiety to elicit aerotactic responses. Mutational lesions (indicated by black diamonds) can enhance the CW-inducing PAS interaction in several different ways. CW-biased mutations in the PAS and AS-2/SD segments could conceivably affect the interacting surfaces directly, whereas CW-biased mutations in the AS-1/connector portion of HAMP probably alter the geometry or flexibility of the Aer molecule to favor the CW interaction. Different mutational lesions in many of the same Aer structural elements destabilize the Aer molecule, leading to rapid degradation and apparent null defects. We propose that these mutant proteins are unstable because their PAS domains cannot achieve native structure, perhaps because they fail to bind FAD. The nonnative PAS domain is the primary target for proteolytic cleavage of Aer molecules that fail to mature. Maturation requires a structural interaction between the nascent PAS domain and the AS-2 segment of HAMP. Null mutations in the PAS and AS-2 segments (indicated by unfilled circles) may directly abrogate that interaction. Null mutations in the F1, AS-1, and connector segments (unfilled circles) may influence PAS/AS-2 interactions indirectly, for example, through altered membrane interaction, geometry, or flexibility of the molecule. Null mutations in the proximal signaling domain (indicated by a shaded circle) also destabilize the Aer molecule, but to a less drastic extent. These lesions could distort the structure or dynamics of the adjacent AS-2 segment and thereby affect the efficiency of the PAS maturation process. WT, wild type.