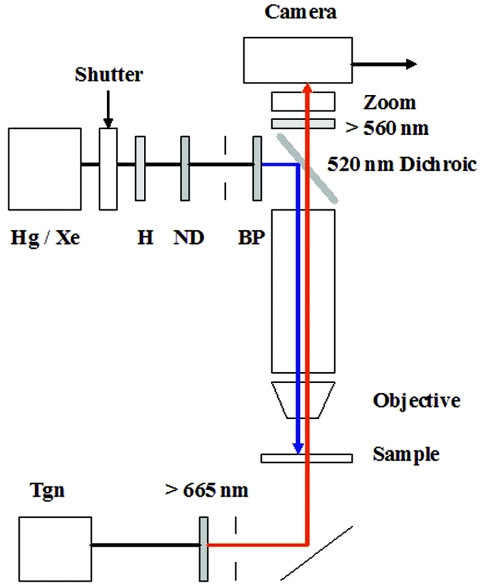
FIG. 1.
Microscope setup for blue-light assays. Cell samples (10 μl) were applied to bridged coverslips placed on the stage of a Nikon Optiphot microscope and observed under phase contrast (40×, 0.85 numerical-aperture fluorite objective), infrared illumination from a tungsten (Tgn) lamp (12 V, 50 W) selected by a 665-nm long-pass filter and reflected up towards the sample by a silvered mirror. A 100-W mercury (Hg) or xenon (Xe) lamp was used to stimulate the cells. The light pulse was heat filtered (H) and its duration was controlled by an electronic shutter (see reference 25 for details). Diaphragms, positioned after the filters, were used to obtain Kohler illumination for both the monitoring and excitation beams. A 520-nm dichroic mirror reflected the excitation beam down onto the sample, while a 560-nm long-pass barrier filter blocked the beam from the camera. Image magnification was adjusted by a zoom lens positioned in front of the camera. The wavelength of the excitation beam was controlled by band pass (BP) (440 ± 5 nm, 440 ± 15 nm, 450 ± 25 nm, 410 ± 5 nm, 470 ± 5 nm, 500 ± 5 nm) and attenuated by neutral density (ND) filters (1/2, 1/4, 1/8) positioned in series before the dichroic mirror. All filters were purchased from Omega Optical (Brattleboro, VT). The study of the E. coli hem mutant light responses used similar optics with two differences: a 50-W rather than 100-W Hg lamp was used for the excitation beam, with a broader (396- to 450-nm) band-pass filter (51).