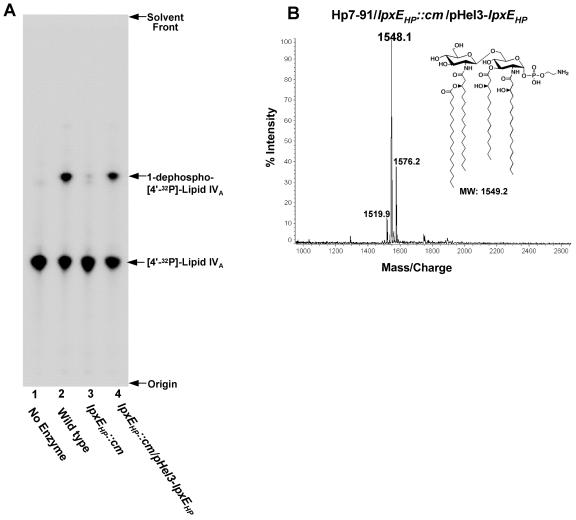
FIG. 5.
Reconstitution of 1-phosphatase activity in membranes of an H. pylori Hp7-91/lpxEHP mutant by the introduction of an lpxEHP-complementing plasmid (A) and MALDI-TOF mass spectrometry of lipid A obtained from the complemented Hp7-91/lpxEHP::cm mutant (B). Membranes isolated from the indicated H. pylori strains were assayed for 1-phosphatase activity under conditions described in Materials and Methods. Protein concentrations were 1.0 mg/ml, and the assays were carried out for 1 h at 30°C. Each reaction mixture contained 5 μΜ of [4′-32P]lipid IVA substrate. The reaction products were separated by TLC and detected by phosphorimager analysis. Removal of the 1-phosphate group was indicated by conversion of the [4′-32P]lipid IVA substrate to a faster-migrating species (56). (B) The lipid A of Hp7-91/lpxEHP::cm transformed with pHel3-lpxEHP was isolated and fractionated by DEAE-cellulose chromatography using published protocols (40). The lipid A sample was analyzed by MALDI-TOF mass spectrometry in the negative-ion mode. The data shown are from lipid A species found to elute in the flowthrough and wash fractions.