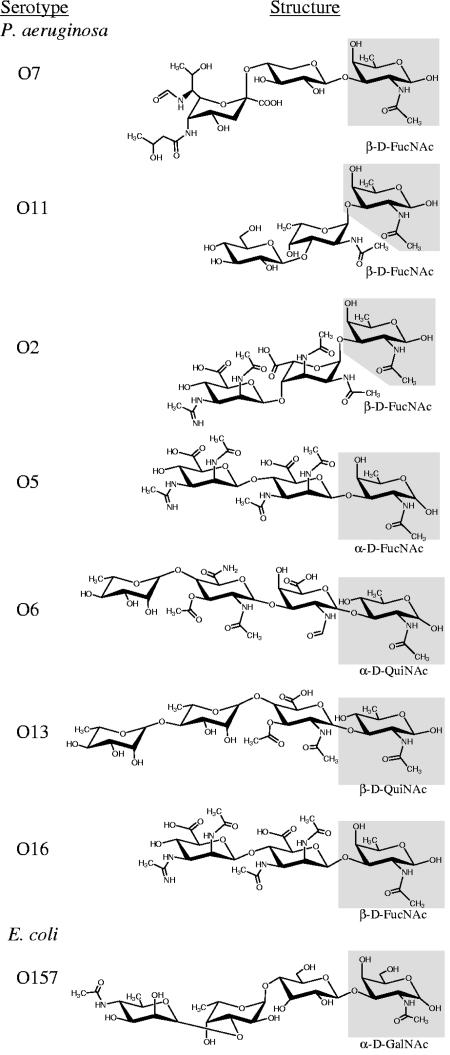
FIG. 1.
Comparative structural analysis of O-antigen subunits previously shown to be compatible for use in pilin glycosylation (14). Chair conformations of O repeats were drawn by using ChemDraw 9.0 based on structures presented by Knirel and Kochetkov (28) and/or Perry and MacLean (38). Presumably, the sugar at the reducing end (highlighted in gray) is bound to Und-PP prior to pilin glycosylation, O-antigen polymerization, and ligation to the core-lipid A. PilO from strain 1244 (serotype O7) was found to mediate the O linkage between carbon-1 of the moiety at the reducing terminus (β-d-FucNAc) to Ser148 of pilin (7, 10). All O repeats tested for use as the glycan were likely bound to pilin in this fashion.