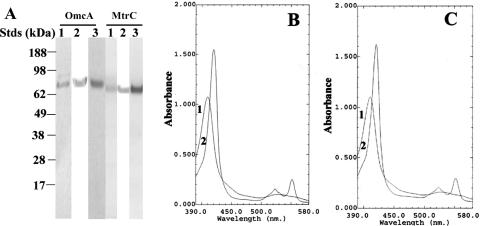
FIG. 1.
Purified OmcA and MtrC. (A) SDS-PAGE (8 to 12% [wt/vol] acrylamide gradient) demonstrating electrophoretic purity of purified (1.0 μg) OmcA (left) and MtrC (right) following visualization with GelCode blue stain (lanes 1), immunoblot assays with antibodies against the V5 epitope tag (lanes 2), and heme staining (lane 3). Migration positions of protein standard (Stds) are indicated at the left. (B and C) Absorption spectra of oxidized proteins (80 μg/ml) (curve 1) and following reduction with dithionite (curve 2) for either OmcA (B) or MtrC (C) in 50 mM HEPES (pH 7.5), 150 mM NaCl, and 1% (wt/vol) OGP. Following purification of 10 mg of MtrC, nine additional proteins (<0.1 mg total) were identified by LC-FTICR and MALDI-TOF. In order of peptide abundance, they were the ATP synthase F1 beta subunit (SO4747), a putative outer membrane porin (SO3896), the ATP synthase F1 alpha subunit (SO4749), the MotA/TolQ/ExbB proton channel (SO1825), putative long-chain fatty acid transport protein (SO3099), MSHA pilin MshA (SO4105), putative outer membrane protein OmpK (SO1215), a conserved hypothetical protein (SO3343), and outer membrane protein A (SOA0114).