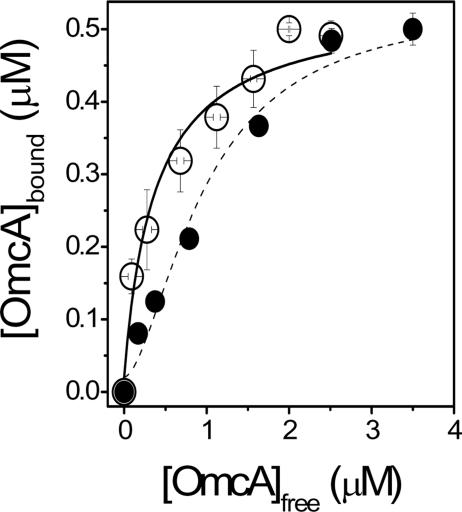
FIG. 8.
Cooperative association between OmcA and MtrC. Binding isotherms are shown for association between OmcA and FlAsH-labeled MtrC, using OmcA without a tetracysteine tag (•) or with a tetracysteine tag (○) that promotes dimer formation, i.e., (OmcA)2. Curves represent respective fits to the Hill equation (see the last equation in Materials and Methods). For monomeric OmcA, Kd = 1.0 ± 0.1 μM and n = 1.7 ± 0.2. Upon dimerization, Kd = 0.4 ± 0.1 μM and n = 1.0 ± 0.1. In all cases, experimental conditions involved 0.5 μM FlAsH-labeled MtrC in 50 mM HEPES (pH 7.5), 140 mM KCl, and 2% OGP.