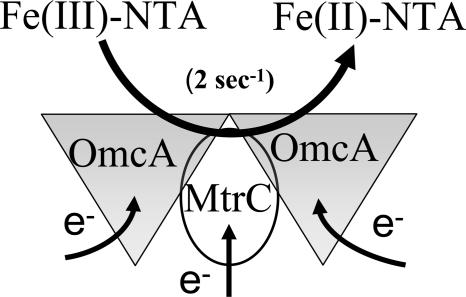
FIG. 9.
Model of the protein complex between OmcA and MtrC. An oligomeric complex involving outer membrane decaheme cytochromes OmcA and MtrC permits coupling between dehydrogenases in the inner membrane that couple the oxidation of NADH and a range of different substrates through classical oxidative pathways involving quinol intermediates and periplasmic proteins and the reduction of Fe(III)-NTA by either OmcA or MtrC. Rates of Fe(III)-NTA reduction are rate limiting (2 s−1), as evidenced by the virtually identical rate constants obtained from direct measurements of OmcA or MtrC oxidation (Fig. 2) relative to that associated with the NADH-dependent reduction of Fe(III)-NTA (Fig. 4).