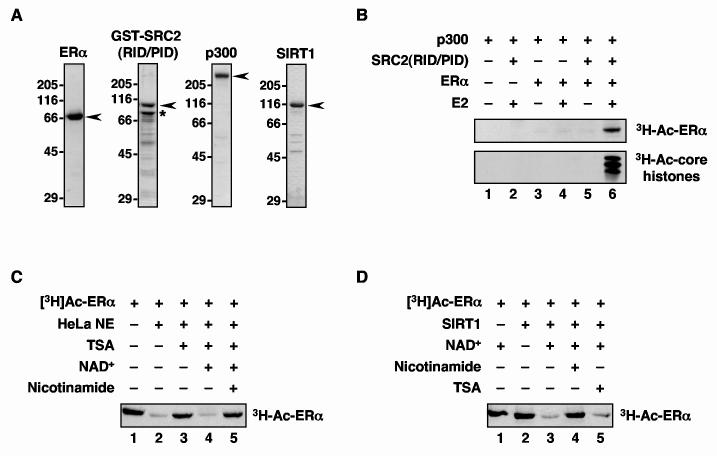
Fig. 1.
ERα is acetylated by p300 and deacetylated by TSA- and nicotinamide-sensitive deacetylases. (A) Polyacrylamide-SDS gel analyses of purified recombinant ERα, GST-SRC2(RID/PID), p300, and SIRT1 stained with Coomassie brilliant blue R-250. Size markers in kDa are shown. The asterisk for the GST-SRC2(RID/PID) sample indicates a major breakdown product with minor breakdown products below. (B) p300 acetylates ERα and nucleosomal core histones in an E2- and SRC-dependent manner. ERα and salt-dialyzed chromatin were incubated with p300 in the presence of GST-SRC2(RID/PID), E2, and [3H]-acetyl CoA. The reactions were analyzed by polyacrylamide-SDS gel electrophoresis with subsequent fluorography. (C) ERα is deacetylated by TSA- and nicotinamide-sensitive deacetylases. [3H]-ERα was incubated with HeLa cell nuclear extract (HeLa NE) in the presence or absence of NAD+, TSA, or nicotinamide as indicated. The reactions were analyzed by polyacrylamide-SDS gel electrophoresis with subsequent fluorography. (D) ERα is deacetylated by the NAD+-dependent deacetylase SIRT1. The assays were set up as in (C) except that purified recombinant SIRT1 was used in place of the HeLa cell NE.