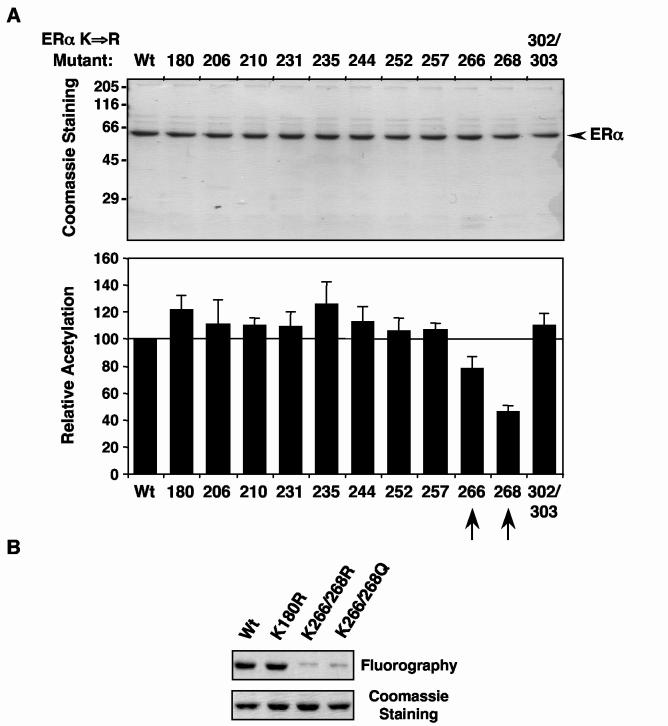
Fig. 3.
Mutation of Lys266 and Lys268 in ERα reduces acetylation by p300. (A) Acetylation assays with ERα lysine mutants. Purified FLAG-tagged wild type and Lys to Arg single-point mutant ERαs were assayed for acetylation by p300 in the presence of GST-SRC2(RID/PID) and E2 as described for Fig. 1B. top, Polyacrylamide-SDS gel analysis of the purified ERα proteins with subsequent staining using Coomassie brilliant blue R-250 to confirm equal protein amounts. Size markers in kDa are shown. bottom, Summary of the results from the acetylation assays. The ERα bands were excised from the gels after fluorography and quantified by liquid scintillation counting. The acetylation level of each lysine point mutant was expressed relative to wild type. Each bar represents the mean plus the SEM from at least three different experiments. (B) Acetylation assays with Lys to Arg 266/268 double-point mutant ERαs. Wild type and mutant ERαs were assayed for acetylation by p300 in the presence of GST-SRC2(RID/PID) and E2 as described for Fig. 1B. top, Polyacrylamide-SDS gel analysis of the purified ERα proteins with subsequent fluorography. bottom, The same gel stained using Coomassie brilliant blue R-250 to confirm equal protein amounts.