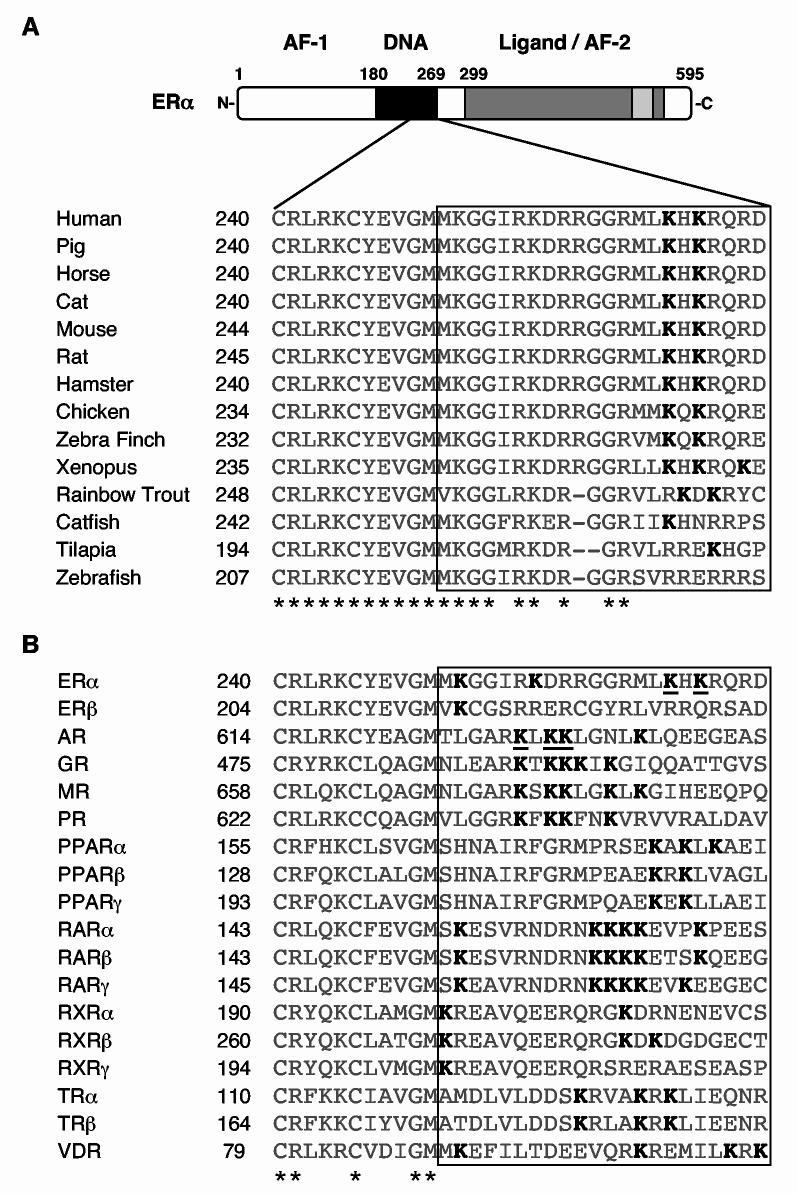
Fig. 5.
Alignment of the amino acid sequences surrounding Lys266 and Lys268 in human ERα with corresponding regions from other ERαs and other nuclear receptors. Sequence alignment of the C-terminal extension (CTE) of the human ERα DBD with the corresponding region of ERs from other species and other nuclear receptors. The alignments were anchored at the last conserved cysteine residue in the second zinc finger of the DBD (underlined C). The boxed region demarcates the amino-terminal portion of the CTE (i.e., those residues located within +11 to +32 of the aforementioned cysteine residue; note that the full CTE, as defined by Melvin et al. (17), extends to +47). Asterisks indicate residues that are conserved in all the receptors shown. (A) Alignment of ERs. The lysine residues (K) shown in bold correspond to Lys266 and Lys268 in human ERα. (B) Alignment of human nuclear receptors. All of the lysine residues (K) in the boxed region are shown in bold. K266 and K268 of ERα, and K630, K632, and K633 of AR are underlined.