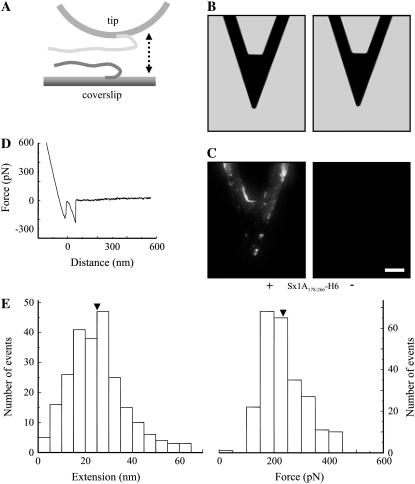
FIGURE 4.
The SNARE domain of Sx1A is sufficient for interaction with Sb2. (A) Cantilevers incubated with Sx1A178-266-H6 (+), a truncated form of Sx1A encoding for rat sequence aa 178–266 and containing SNARE domain, but lacking the remaining N-terminal part of the Sx1A molecule, were successfully functionalized as indicated by the positive immunoreactivity (C) when compared to the control cantilevers where Sx1A178-266-H6 (−) was not attached to the cantilever. (B) Bright-field images of cantilevers that were subjected to indirect immunochemistry in C. (D) The retraction part of a typical force-distance curve using a truncated Sx1A178-266-H6 functionalized tip and a Sb2-H6 functionalized coverslip. (E) Distributions of the extensions and forces at rupture recorded from the interactions between Sx1A178-266-H6 functionalized tips and Sb2-H6 functionalized coverslips indicate that the SNARE domain of Sx1A is sufficient for interactions with Sb2, whereas the remaining part of Sx1A (aa 1–177) is not necessary for these intermolecular interactions to occur (compare with Fig. 1, F and G). Arrowheads in E indicate mean values. Drawing in A is not to scale. Retraction velocity, 1.6 μm/s. Scale bars in B and C, 30 μm.