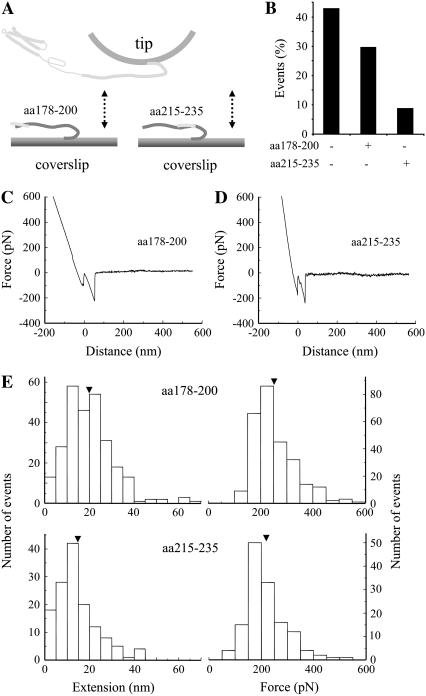
FIGURE 5.
Sb2 and Sx1A are zippered. Sb2 functionalized coverslips were preincubated with peptides encoding for a portion of rat Sx1A molecule, either aa 178–200 or aa 215–235 (A). Force spectroscopy (double arrow) reveals that the number of interactions between Sx1A and Sb2 is reduced in conditions where peptides were preincubated with Sb2 functionalized coverslips (B). The retraction part of typical force-distance curves using a Sx1A-H6 functionalized tip and a Sb2-H6 functionalized coverslip preincubated with either aa 178–200 (C) or aa 215–235 peptides (D). (E) Distributions of the extensions and forces at rupture recorded from interactions between Sx1A-Sb2 in the presence of cognate peptides. Arrowheads in E indicate mean values. Retraction velocity, 1.6 μm/s. Drawings in A are not to scale.