Figure 13.
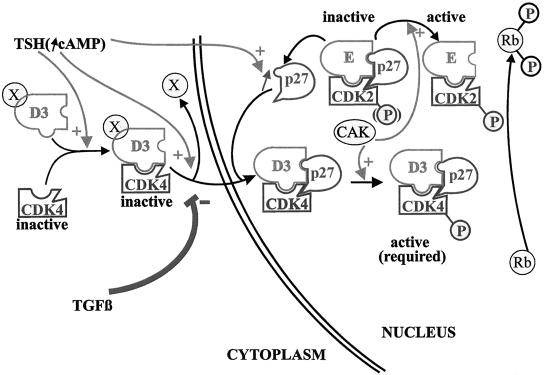
The subcellular localization of cyclin D3 and cdk4 integrates the antagonistic cell cycle effects of TSH and TGFβ. TSH does not stimulate cyclin D3 accumulation, but it assembles cyclin D3–cdk4 complexes, enhances the levels of p27kip1, and induces the nuclear translocation of cyclin D3–cdk4, which correlates with the exposition of a cyclin D3 epitope (depicted by the displacement of the X element). Cyclin D3–cdk4 complexes are stabilized in the nucleus by their binding to p27kip1, which thus might serve as a nuclear anchor. This nuclear translocation of cdk4 is assumed to be required for its phosphorylation by nuclear CAK and for access to Rb. TGFβ does not inhibit the assembly of cyclin D3–cdk4 complexes, nor p27kip1 accumulation, but it prevents the epitope unmasking of cyclin D3 and the nuclear translocation of cyclin D3–cdk4. Consequently these complexes are prevented from encountering p27kip1 and thus sequestering it away from the initial pool of nuclear cdk2 complexes. Cyclin D3–cdk4 is thus maintained in an inactive state by its cytoplasmic location, and nuclear cdk2 is inhibited by its association with p27kip1. The further import of cdk2 from the cytoplasm induced by TSH but repressed by TGFβ as a likely indirect consequence of this mechanism, is not depicted.