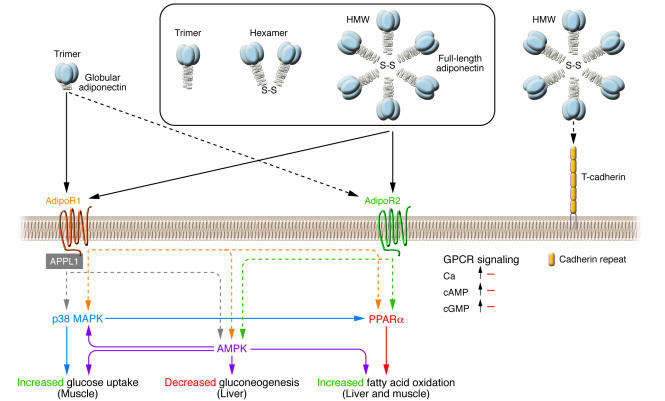
Figure 3. Signal transduction by adiponectin receptors.
Globular adiponectin exists as a trimer, whereas full-length adiponectin exists as at least 3 species of multimers: an LMW trimer, an MMW hexamer, and an HMW multimer. Suppression of AdipoR1 by RNA interference markedly reduces globular adiponectin binding, whereas suppression of AdipoR2 by RNA interference largely reduces full-length adiponectin–specific binding (21, 84). The dotted line between AdipoR2 and globular adiponectin reflects that AdipoR2 is a relatively low-affinity receptor for globular adiponectin. AdipoR1 and AdipoR2 do not seem to be coupled with G proteins, since overexpression of AdipoR1/R2 has little effect on cAMP, cGMP, and intracellular calcium levels, but instead these receptors activate unique sets of signaling molecules such as PPARα, AMPK, and p38 MAPK. In C2C12 myocytes overexpressing AdipoR1/R2, adiponectin stimulates PPARα, AMPK, and p38 MAPK activation, glucose uptake, and fatty-acid oxidation (84). Suppression of AMPK or PPARα partially reduces adiponectin-stimulated fatty-acid oxidation, and suppression of AMPK or p38 MAPK partially reduces adiponectin-stimulated glucose uptake. In hepatocytes overexpressing AdipoR1/R2, adiponectin stimulates PPARα or AMPK and fatty-acid oxidation (84). Suppression of AMPK or PPARα in these hepatocytes partially reduces adiponectin-stimulated fatty-acid oxidation. Moreover, treatment with adiponectin reduces plasma glucose levels and molecules involved in gluconeogenesis in the liver, and dominant-negative AMPK partly reduces these effects.These data support the conclusion that AdipoR1 and AdipoR2 serve as receptors for globular and full-length adiponectin and mediate increased AMPK, PPARα ligand activities, p38 MAPK, and adiponectin-induced biological functions.T-cadherin is capable of binding adiponectin but is thought to have no effect on adiponectin cellular signaling, since T-cadherin lacks an intracellular domain (86). Interaction of APPL1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif 1) with AdipoR1 appears to play important roles in adiponectin signaling and adiponectin-mediated downstream events such as lipid oxidation and glucose uptake (88). S-S, disulfide bond. Adapted with permission from Endocrine Reviews (21); copyright 2005, The Endocrine Society.