Abstract
Purpose
To determine whether radiation therapy (RT) of patients with Wilms tumor of favorable histology (FH) prevented flank recurrence and thereby improved the survival outcome.
Methods and Materials
Recurrence and mortality risks were compared among groups of patients with stage I-IV/FH Wilms tumor enrolled in the 3rd (n=1640) and 4th (n=2066) National Wilms Tumor Study Group (NWTSG) studies.
Results
Proportions of patients with flank recurrence were 0/513=0.0% for 20 Gy, 12/805=1.5% for 10 Gy and 44/2388=1.8% for no flank RT (p-trend=0.001 adjusted for stage and doxorubicin); for intra-abdominal (including flank) recurrence they were 5/513=1.0%, 30/805= 3.7% and 58/2388=2.4%, respectively (p-trend=0.02 adjusted). Survival percentages at 8 years following intra-abdominal recurrence were 0/5=0% for 20 Gy, 10/30=33% for 10 Gy and 34/58=56%) for no RT (p-trend=0.0001). NWTS-4 discontinued use of 20Gy RT and the 8 year flank recurrence risk increased to 2.1% from 1.0% on NWTS-3 (p=0.013). However, event-free survival was unaltered (88% vs. 86%, p=0.39) and overall survival was better (93.8% vs. 90.8%, p=0.036) on NWTS-4.
Conclusions
Due partly to lower post-recurrence mortality among non-irradiated patients, prevention of flank recurrence by RT did not improve survival. It is important to evaluate entire treatment policies with regard to long-term outcomes.
INTRODUCTION
Radiation therapy (RT) for Wilms tumor (WT) during the 1940's is credited with increasing to nearly 50% the cure rates of 15%-30% observed in the 1930's with nephrectomy alone.(1;2) Addition of single agent chemotherapy in the 1950's further improved 2 year survival to 60%-80%.(3;4) When the National Wilms Tumor Study Group (NWTSG) began operation in North America in 1969, standard treatment consisted of nephrectomy, 18-40 Gy RT to the operative bed (flank) and adjuvant dactinomycin or vincristine. Motivated by increasing concern over chronic RT toxicity, the first two NWTSG studies sought to determine whether chemotherapy might substitute for RT in selected patients. They demonstrated excellent results without RT for patients with WT of favorable histology (FH) whose disease was confined to the kidney and completely resected (stage I).(5-7) The third study (NWTS-3) showed that RT could be omitted also in patients with FH disease that extended beyond the kidney but was completely removed (stage II), provided they received both dactinomycin and vincristine.(8) Treatment mandated for patients with residual (stage III) or metastatic (stage IV) FH disease on NWTS-4 was a dose of only 10 Gy RT to the flank combined with doxorubicin (DOX) in addition to dactinomycin and vincristine.(9;10) The 2 year event-free survival (EFS) of 85% was unexpectedly low, however, for patients with stage II/FH disease compared to stage III/FH (90%). Intra-abdominal recurrence was particularly elevated (16%) in patients whose stage II/FH disease had spilled and concern arose that they may have been under treated.(11)
In Europe the International Society for Pediatric Oncology (SIOP) employed prenephrectomy RT and chemotherapy to reduce tumor mass and thereby lessen the risk of spillage during surgery.(12-14) The 6th study (SIOP-6), which used chemotherapy alone pre-operatively and randomized patients with residual stage II node negative disease post-nephrectomy to 20 vs. 0 Gy RT, stopped randomization and offered all such patients 20 Gy RT when 6 abdominal recurrences occurred in the no RT and none in the 20 Gy group.(15) An anthracycline was substituted for RT in subsequent protocols.(16)
In spite of progress made by NWTS and SIOP, the optimal use of RT to the flank or wider abdominal fields remains an open question.(17) The primary motivation for its use is to prevent a relatively rare event, flank recurrence, which tends to occur shortly after treatment. Evidence of deleterious effects in promoting second cancers, cardiomyopathies (in patients also receiving DOX), developmental abnormalities and adverse pregnancy outcomes, by contrast, may take decades to develop.(18-21) There is no guarantee that use of RT to prevent flank recurrence will improve long term survival. The present study was undertaken to determine if reduction in use of RT between NWTS-3 and NWTS-4 had adversely affected survival outcomes for patients with FH disease. A parallel evaluation of DOX was undertaken earlier for patients with stage II-III/FH disease.(22)
METHODS AND MATERIALS
Patients
Eligibility criteria for NWTS-3 and NWTS-4 included age under 16 years at diagnosis of WT, clear cell sarcoma or malignant rhabdoid tumor of kidney and nephrectomy before treatment with RT or chemotherapy. Information regarding diagnosis, staging, treatment and therapeutic outcomes has been published.(8-10) NWTS-3 utilized a factorial design whereby patients with stage II/FH disease were randomized simultaneously to no flank RT or 20 Gy and to treatment with or without DOX in addition to dactinomycin and vincristine. Those with stage III/FH disease were randomized to 10 or 20 Gy RT and to chemotherapy with or without DOX. The NWTS-4 randomization involved only duration and scheduling of chemotherapy. Because neither influenced outcomes in children with FH disease, however, these factors are ignored in the sequel.(9) “Followed” patients, i.e., eligible patients who were treated and had data submitted per protocol but who were not randomized for various reasons, were included together with randomized patients in the present observational study to maximize statistical precision and minimize selectivity.(8) No exclusions were made for protocol deviations that occurred due to a change in stage or histology. Although very few patients with stage I/FH disease received RT or DOX, they accounted for nearly one third of the flank recurrences and were included to enhance the statistical power of comparisons of recurrence rates between subgroups.
Between October, 1979 and August, 1986, 1793 patients with stage I-IV WT were entered on NWTS-3. Fifty-seven were excluded for lack of baseline surgery or pathology records, 11 for tumor in a solitary or fused kidney, 3 for initial treatment not on a protocol regimen and 88 for anaplastic histology. Between August, 1986 and September, 1994, 2345 patients with stage I-IV WT were entered on NWTS-4. One hundred eighteen were excluded for lack of baseline records, 16 for tumor in a solitary or fused kidney, 11 for non-protocol treatment and 148 for anaplastic histology. This left 1640 NWTS-3 and 2066 NWTS-4 patients for statistical analysis, of whom 952 (26%) were “followed”.
The NWTS-3,4 protocols were approved by the Institutional Review Board of each institution registering patients. Informed consent was obtained from the parents of all patients prior to participation in the study.
Treatment Outcomes
Treatment factors were correlated with each of four endpoints: (i) recurrence in the original tumor bed (flank); (ii) intra-abdominal recurrence, in the flank or beyond the flank but excluding hematogenous metastasis to the liver and new disease in the contralateral kidney; (iii) EFS, where the endpoint is recurrence, metastasis, disease progression or death without disease; and (iv) overall survival (OS). Patients were considered at risk of flank and intra-abdominal recurrence only until the initial relapse event since treatment generally changed at that point. All deaths were counted in the EFS and OS analyses since both therapeutic and toxic effects of RT on these major endpoints were at issue.
Sources of Data
Data submitted on surgical checklists and narratives were abstracted by staff and reviewed by NWTSG surgeons. Slides were reviewed at the Pathology Center to confirm the diagnosis of FH WT. RT fields and doses abstracted from checklists were reviewed by NWTSG radiation therapists. Sites of disease progression or recurrence and causes of death abstracted from clinical records were reviewed by study oncologists. Stage was assigned by the institution, usually after Pathology Center input.
Statistical Methods
EFS and OS curves and standard errors (SE) were estimated by actuarial methods, as were cumulative recurrence risks.(23) Comparisons between patient subgroups, often adjusted for other factors by stratification, were made with the log rank test.(24) Tests for trend in RT dose and estimates of relative risk (RR) with 95% confidence intervals (CI) were based on the Cox model.(25)
Role of the Funding Source
The funding source approved the initial NWTS-3,4 protocols but otherwise had no role in study design, in collection, analysis or interpretation of data or in writing the article. The corresponding author had full access to all study data and responsibility for the decision to submit for publication.
RESULTS
Treatments
Patients were assigned to one of three categories according to the recorded amount of flank RT: none (0 Gy), 10 Gy (0.1-14.9 Gy) and 20 Gy (15+ Gy). Some patients received irradiation to the entire abdomen. Patients were also classified by whether or not primary treatment included DOX. Numbers of patients in each category and mean flank RT doses are shown in Table 1 by study and stage. The overwhelming majority received protocol treatment for their stage (see Patients). For stage IV disease due to pulmonary metastasis this included 12 Gy to both lungs plus flank RT and DOX, although the flank RT was sometimes omitted for those with local stage I or II disease.
Table 1.
Use of flank radiation therapy and doxorubicin by study and stage
| No RT* | 10 Gy RT | 20 Gy RT | DOX† | |||||
|---|---|---|---|---|---|---|---|---|
| Study | Stage | No. Pts. | % of Pts. | % of Pts. | Mean±SD Dose (Gy) | % of Pts. | Mean±SD Dose (Gy) | % of Pts. |
| 3 | I | 689 | 98.7 | 0.1 | 3.6±0.0 | 1.2 | 28.2±8.1 | 0.3 |
| II | 378 | 58.5 | 6.3 | 10.1±1.9 | 35.2 | 20.8±2.5 | 40.7 | |
| III | 377 | 1.9 | 50.4 | 10.4±0.7 | 47.7 | 21.1±3.8 | 64.5 | |
| IV | 196 | 4.1 | 10.2 | 11.0±1.2 | 85.7 | 22.2±4.8 | 99.5 | |
| 4 | I | 825 | 99.8 | 0.2 | 10.7±0.2 | 0.0 | --- | 0.2 |
| II | 580 | 98.4 | 1.0 | 10.8±0.0 | 0.5 | 28.0±8.4 | 1.0 | |
| III | 434 | 5.3 | 92.4 | 10.7±0.7 | 2.3 | 19.7±1.9 | 99.1 | |
| IV | 227 | 24.2 | 70.9 | 10.7±0.6 | 4.8 | 27.6±7.9 | 100.0 | |
RT = radiation therapy
DOX = doxorubicin
On NWTS-3 overall, 724 patients (44%) received flank RT as part of initial treatment, 489 (68%) of them at 20 Gy, and 594 (36%) received DOX. On NWTS-4 only 594 patients (29%) received RT, nearly all at 10 Gy, and 665 (32%) received DOX. Of 55 NWTS-4 patients with stage IV disease and no RT to the flank (Table 1), 44 received pulmonary RT. Percentages of irradiated patients who received whole abdominal RT by dose category were 23% for 10 and 18% for 20 Gy on NWTS-3, and 29% for 10 and 46% for 20 Gy on NWTS-4. The few patients who received non-protocol treatment usually had a change in stage or histology (unfavorable to favorable) after starting treatment.
Although the only primary treatment differences between NWTS-3 and NWTS-4 involved use of RT and DOX, there were substantial differences in post-relapse therapy. Using retrieval therapy data available for relapsed patients of all stages and histologies, for example, the percentages who received etoposide were 28% vs. 77%, cis-platinum 31% vs. 12%, and ifosfamide 13% vs. 52% for NWTS-3 (n=185) vs. NWTS-4 (n=212), respectively.
Outcomes by Stage for NWTS-3 vs. NWTS-4
The stage distribution differed somewhat between studies (Table 1), with NWTS-4 having a higher percentage (580/2066=28%) of tumors assigned stage II than NWTS-3 (378/1640=23%, p=0.01). This was primarily due to “upstaging”, as permitted by protocol, of NWTS-4 patients who had tumor in the renal vein or sinus beyond the renal hilar plane but otherwise fulfilled stage I criteria.(26)
For each stage the risk of flank recurrence was higher on NWTS-4 than NWTS-3 and the summary relative risk adjusted for stage was RR=2.1 (CI=1.2-3.8, p=0.013, Table 2). The slight increase for patients with stage I disease, however, was not statistically significant on its own (p=0.30). The risk of intra-abdominal recurrence also was higher on NWTS-4 (RR=1.7, CI=1.1-2.7, p=0.014, adjusted). By contrast, there was no difference in EFS (RR=0.93, CI=0.78-1.11, p=0.39, adjusted) and mortality was the same or lower on NWTS-4 for every stage (RR=0.77, CI=0.61-0.98, p=0.036, adjusted). When restricted to patients who received DOX, virtually all with stage III/IV disease, the mortality RR was reduced to 0.72 (CI=0.51, 1.02, p=0.066, adjusted). Figure 1 shows the OS curves for each study. The numbers of patients alive and followed at 5 and 10 years, respectively, were 1,427 and 1,283 for NWTS-3 and 1,696 and 772 for NWTS-4.
Table 2.
Numbers of relapses and deaths, and percentages relapsed or died at 8 Years (±S.E.) by study and stage
| Flank Recurrence | Intra-abdominal Recurrence | Relapse (or Death) | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Stage | No. Pts. | % Recur at 8 yrs | No. Pts. | % Recur at 8 yrs | No. Pts. | % Relapse at 8 yrs | No. Pts. | % Died at 8 yrs. |
| 3 | I | 6 | 0.9±0.4 | 6 | 0.9±0.4 | 64 | 8.5±1.1 | 28 | 3.3±0.7 |
| II | 6 | 1.7±0.7 | 10 | 2.8±0.9 | 44 | 10.7±1.6 | 31 | 7.1±1.3 | |
| III | 3 | 1.0±0.5 | 10 | 3.0±0.9 | 75 | 19.8±2.1 | 50 | 13.0±1.7 | |
| IV | 0 | 0.0±0.0 | 3 | 1.1±1.1 | 50 | 23.5±3.0 | 42 | 18.9±2.8 | |
| 4 | I | 12 | 1.5±0.4 | 17 | 2.2±0.5 | 69 | 8.3±1.0 | 27 | 3.4±0.7 |
| II | 18 | 3.6±0.8 | 22 | 4.1±0.9 | 95 | 16.7±1.5 | 36 | 6.1±1.0 | |
| III | 6 | 1.5±0.5 | 12 | 3.0±0.9 | 47 | 11.1±1.5 | 30 | 6.9±1.2 | |
| IV | 5 | 2.5±1.1 | 13 | 6.2±1.7 | 46 | 19.6±2.7 | 35 | 15.0±2.4 | |
1.
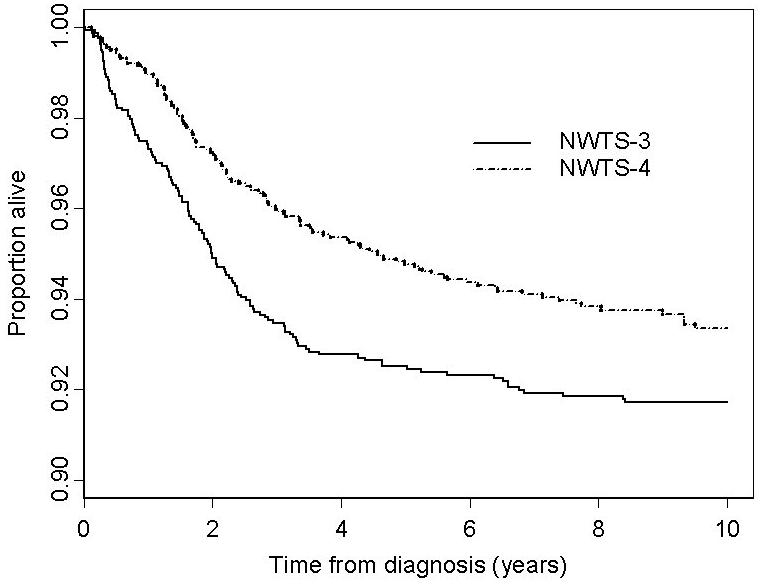
Overall survival curves for patients with favorable histology Wilms tumor on NWTS-3 and NWTS-4
RT Effects on Treatment Outcomes
Results of analyses with RT dose category as a predictor of flank and intra-abdominal recurrence and of EFS are shown in Table 3. The RR and CI were adjusted by joint stratification on stage and DOX, with which RT was highly associated (Table 1). Although the 513 patients receiving 20 Gy RT had higher stage disease, no flank recurrence occurred among them. The number expected under the hypothesis of no difference between RT categories, using the stratified log rank procedure, was 7.4. Administration of 20 Gy RT also had a statistically significant effect on intra-abdominal recurrence more generally (5 observed vs. 16.9 expected, p = 0.001), with a fourfold reduction in risk compared to 10 Gy or no RT (Table 3). By contrast, there were no differences among the 20 Gy, 10 Gy and no RT categories in EFS percentage, where the numbers of events were much greater. However, there were differences in the types of events that contributed to EFS, with deaths due to toxicity or other causes in the absence of disease accounting for 22/101 (22%) of the events in patients receiving 20 Gy vs. 15/121 (12%) for 10 Gy and 29/268 (11%) for no RT (p=0.01). For lung metastasis as an event type, by contrast, the percentages were nearly identical: 51/101 (50%) for 20 Gy, 55/121 (45%) for 10 Gy and 133/268 (50%) for no RT (p=0.95)
Table 3.
Relative risks and confidence Intervals for flank recurrence, Intra-abdominal recurrence and relapse/death by use of flank radiation therapy
| RT* dose | Total Pts. | Flank Recurrence | Intra-abdominal Recurrence | Relapse (or Death) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | RR† | CI‡ | No. | RR | CI | No. | RR | CI | ||
| None | 2388 | 44 | 1.00 | 58 | 1.00 | 268 | 1.00 | |||
| 10 Gy | 805 | 12 | 0.61 | 0.01-2.21 | 30 | 0.98 | 0.41-2.31 | 121 | 0.85 | 0.58-1.25 |
| 20 Gy | 513 | 0 | 0.00 | NA | 5 | 0.23 | 0.07-0.76 | 101 | 0.99 | 0.70-1.40 |
| p-trend = 0.001 | p-trend = 0.02 | p-trend = 0.56 | ||||||||
RT = radiation therapy
RR = relative risk
CI confidence interval RR and CI adjusted for stage and DOX.
The difference in use of RT between studies explained statistically the increase in flank recurrence on NWTS-4. After adjustment for RT in addition to stage, the RR for NWTS-4 vs. NWTS-3 was reduced from 2.1 to 1.3 (CI=0.7-2.4; p=0.35).
After joint stratification on study, stage and DOX, the relative risk of death for patients receiving 20 Gy RT vs. no RT was RR=1.36 (CI=0.81, 2.26, p=0.24) but the small excess of deaths for 20 Gy RT (82 observed vs. 75.3 expected) was not statistically significant. The numbers of deaths ascribed to toxicity or infection were 22 (27%) for 20 Gy, 16 (20%) for 10 Gy and 19 (16%) for no RT, with 4 of the toxic deaths in each RT group occurring in the presence of disease. However, the test for trend was not statistically significant (p=0.08). Second malignant neoplasms accounted for 7 deaths overall, congestive heart failure for 5 and end stage renal disease for 2, but these latter causes accounted for approximately the same percentage (4-6%) of the deaths in each of the three RT groups. Twenty-two deaths that occurred from other and unknown causes, including accidents and violence, were also distributed proportionally across the RT dose categories.
Post-Relapse Mortality
We also compared the survival outcomes for relapsed patients by study and initial treatment. Sixty-six patients who died of toxicity or other causes without having relapsed, 13 patients who died from metastatic disease that failed to respond to initial therapy and one patient whose liver metastasis was discovered at death were excluded from the group of 490 counted as failures in the EFS analysis. This left 410 patients with relapsed WT. Actuarial percentages of patients alive 8 years post relapse were 43% on NWTS-3 and 55% on NWTS-4 (Figure 2). The relative risk of death for NWTS-3 vs NWTS-4 was RR=0.72 (CI=0.54-0.95; p=0.02). When adjusted by stratification on RT, however, post-relapse mortality risks were nearly the same for NWTS-3 and NWTS-4 (RR=0.98, CI=0.70-1.37; p=0.91).
2.
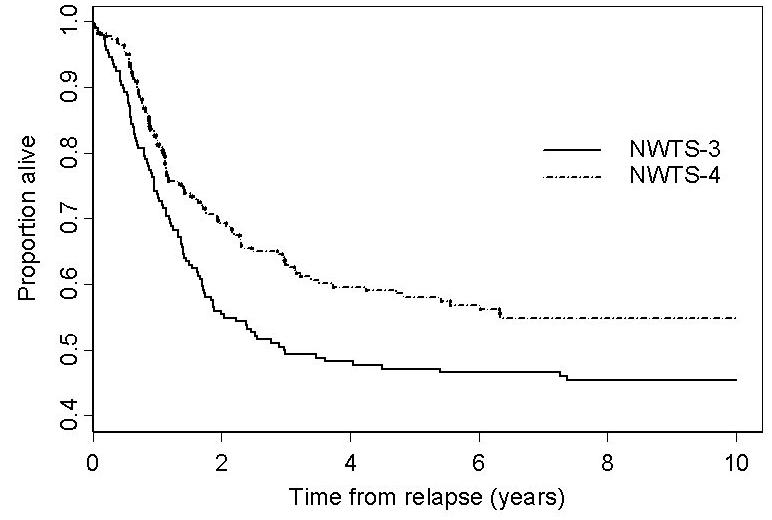
Survival after relapse for relapsed patients with favorable histology Wilms tumor on NWTS-3 and NWTS-4
Post-relapse mortality was higher than expected for patients who received more RT after joint stratification on study, stage and DOX. Observed vs. expected numbers of deaths / numbers of relapsed patients by RT dose category were 85 vs. 90.7 / 237 for no RT, 61 vs. 63.1 / 101 for 10 Gy and 53 vs. 45.1 / 72 for 20 Gy (p-trend=0.04). Due to the randomization employed, most of the information for this adjusted analysis came from NWTS-3 (Table 4). Nearly 90% of deaths following relapse were due to progressive disease, with the percentages of deaths caused by progressive disease ranging from 86% to 91% across the three RT categories. Differences between these categories in survival were even more pronounced when restricted to 93 patients who first relapsed intra-abdominally in the flank or beyond (Table 3). Numbers alive after intra-abdominal recurrence / numbers of patients (actuarial 8 year survival) were 0/5 (0%) for 20 Gy, 10/30 (33%) for 10 Gy and 34/58 (56%) for no RT (p-trend=0.0001). Adjustment for DOX, stage and study using the Cox model actually enhanced the trend while leaving the statistical significance unchanged.
Table 4.
Actuarial survival percentages (±SE) among relapsed patients by study and use of flank radiation therapy
| Study | RT* | No. Pts. | No. of Deaths | P-Value | % Alive at 8 Years | |
|---|---|---|---|---|---|---|
| Observed | Expected† | |||||
| 3 | None | 80 | 32 | 36.1 | .04‡ | 59.8±5.5 |
| 10 Gy | 36 | 20 | 24.5 | 46.9±8.3 | ||
| 20 Gy | 70 | 52 | 43.4 | 24.8±5.3 | ||
| 4 | None | 157 | 53 | 54.7 | .38 | 63.3±4.1 |
| 10 & 20 Gy | 67§ | 42 | 40.3 | 35.3±6.0 | ||
RT = radiation therapy
Adjusted for stage and DOX
Trend test
2 @ 20 Gy
DISCUSSION
Our results confirmed the efficacy of 20 Gy flank RT in prevention of recurrence in patients with FH WT (Table 3). Not one flank recurrence occurred among 513 patients so treated. Patients who received no or 10 Gy RT had a flank recurrence risk of less than 2%, which nevertheless resulted in 56 flank recurrences due to the large number of patients. The treatment effect was greatly diluted, however, when evaluated using more common endpoints. For intra-abdominal (including flank) recurrence the relative risks were closer to one and the statistical significance of the trend test reduced (Table 3). There was no effect of RT on EFS. While the number of deaths (82) observed for patients who received 20 Gy RT was slightly higher than expected (75.3) after adjustment for study, stage and DOX, the difference was not statistically significant.
Analyses of post-relapse survival helped explain the failure of RT to improve survival in spite of its efficacy in preventing intra-abdominal and especially flank recurrence. First, intra-abdominal recurrence is a relatively rare event. Second, most deaths follow metastasis to liver or lung, or are caused by toxicity or other factors. Third, comparable patients who relapsed without having received RT, particularly those who had intra-abdominal recurrence, fared better following retrieval therapy than those who had received RT. Some of the association between prior RT and post-relapse mortality might have been expected from the fact that lower stage patients were both less likely to receive RT (Table 1) and more likely to survive following relapse.(27) However, the association persisted even after joint stratification on study, stage and DOX. In fact, an association between prior RT and post-relapse mortality would be anticipated from the biological variation of individual tumors, for example, variation in histology within the favorable subgroup, and the tendency of cancer therapy to selectively suppress the more responsive tumor types.(28) Patients who relapse following intensive therapy typically have less responsive tumors than do those who relapse on conventional therapy. Tumors that progress during therapy are likely to have developed resistance to the agents used. Patients with such tumors may only be retrieved, if at all, using ever more intensive and toxic therapeutic regimens.
We also compared relapse and survival rates on NWTS-3 and NWTS-4, which differed in their use of DOX and especially RT. In spite of increased flank and intra-abdominal recurrence rates on NWTS-4, EFS was unchanged and OS was significantly better. In view of the increase in the post-relapse mortality RR for NWTS-3 vs NWTS-4 from 0.72 to 0.98 after adjustment for RT, the difference in post-relapse mortality between studies may be attributed statistically to the facts that fewer relapsed patients on NWTS-4 had received RT, that almost all who did received only 10 Gy and that post-relapse mortality worsened with increasing prior RT (Table 4). Considering the documented effects of flank RT on chronic toxicity including second malignant neoplasms and, in combination with DOX, congestive heart failure, one may expect the survival advantage for NWTS-4 patients to continue into the future.(18;20;22) Some of the improvement in survival may have resulted from superiority of the NWTS-4 treatment policy, which withheld RT from low risk patients until needed to treat relapse but routinely employed DOX for stage III disease. Some may have resulted from better retrieval therapy and supportive care. DOX definitely improved EFS, and although there was no clear evidence from our earlier study that it improved OS, fewer deaths were observed after DOX treatment than were expected under the hypothesis of no treatment effect.(22) These uncertainties notwithstanding, our results do not support a change in current policy of NWTSG and other cooperative groups of omitting RT from treatment of patients with stage I-II/FH WT. Our seemingly paradoxical results merit reflection. Strong and clear effects of treatment with 20 Gy RT on flank and intra-abdominal recurrence did not lead to demonstrable improvement in survival. In order for an intermediate endpoint to fully capture treatment effects, event rates for the true endpoint given the past history of the intermediate must be statistically independent of treatment group.(29) If we consider intra-abdominal recurrence or even EFS as the intermediate and OS as the true endpoint, this condition was not met. Since flank RT as part of initial treatment was associated with decreased survival after recurrence or relapse, we cannot use recurrence or EFS to predict RT effects on survival. These results serve as a reminder that the most meaningful evaluations of clinical trial results are those that compare entire treatment policies, from diagnosis to death or cure, with regard to long-term outcomes.
ACKNOWLEDGEMENT
We thank investigators of the Pediatric Oncology Group, the Children's Cancer Group and the health professionals who managed the care of children entered on the National Wilms Tumor Group Studies.
Footnotes
Supported in part by United States Public Health Service grants no. CA42326 and CA54498.
REFERENCES
- 1.Halperin EC, Constine LS, Tarbell NJ, Kun LE. Pediatric Radiation Oncology. 3rd ed. Lippincott, Williams and Wilkins; Philadelphia: 1999. [Google Scholar]
- 2.Gross RE, Neuhauser EBD. Treatment of mixed tumors of the kidney in childhood. Pediatrics. 1950;6:843–852. [PubMed] [Google Scholar]
- 3.Farber S. Chemotherapy in the treatment of leukemia and Wilms' tumor. J Am Med Assoc. 1966;198:826–836. [PubMed] [Google Scholar]
- 4.Cassady JR, Tefft M, Filler RM, et al. Considerations in the radiation therapy of Wilms' tumor. Cancer. 1973;32(3):598–608. doi: 10.1002/1097-0142(197309)32:3<598::aid-cncr2820320312>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.D'Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms tumor - Results of the National Wilms Tumor Study. Cancer. 1976;38(2):633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumor. Cancer. 1978;41(5):1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.D'Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms Tumor - Results of the 2nd National Wilms Tumor Study. Cancer. 1981;47(9):2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.D'Angio GJ, Breslow N, Beckwith B, et al. Treatment of Wilms' tumor: Results of the third National Wilms' Tumor Study. Cancer. 1989;64(2):349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16(1):237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 10.Green DM, Breslow NE, Beckwith JB, et al. Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16(12):3744–3751. doi: 10.1200/JCO.1998.16.12.3744. [DOI] [PubMed] [Google Scholar]
- 11.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg. 2000;229(2):292–297. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kraker J, Weitzman S, Voûte PA. Preoperative strategies in the management of Wilms tumor. Hematol Oncol Clin North Am. 1995;9(6):1275–1285. [PubMed] [Google Scholar]
- 13.Lemerle J, Voûte PA, Tournade MF, et al. Preoperative versus postoperative radiotherapy, single versus multiple courses of actinomycin D, in the treatment of Wilms' tumor. Cancer. 1976;38(2):647–654. doi: 10.1002/1097-0142(197608)38:2<647::aid-cncr2820380204>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Lemerle J, Voûte PA, Tournade MF, et al. Effectiveness of preoperative chemotherapy in Wilms tumor - Results of an International-Society-Of-Paediatric-Oncology (SIOP) clinical-trial. J Clin Oncol. 1983;1(10):604–609. doi: 10.1200/JCO.1983.1.10.604. [DOI] [PubMed] [Google Scholar]
- 15.Tournade MF, Com-Nougué C, Voûte PA, Lemerle, et al. Results of the 6th International-Society-Of-Pediatric-Oncology Wilms-tumor trial and study - A risk-adapted therapeutic approach in Wilms-tumor. J Clin Oncol. 1993;11(6):1014–1023. doi: 10.1200/JCO.1993.11.6.1014. [DOI] [PubMed] [Google Scholar]
- 16.Tournade MF, Com-Nougué C, de Kraker J, et al. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms' tumor in children older than 6 months: Results of the ninth International Society of Pediatric Oncology Wilms' tumor trial and study. J Clin Oncol. 2001;19(2):488–500. doi: 10.1200/JCO.2001.19.2.488. [DOI] [PubMed] [Google Scholar]
- 17.Davies-Johns T, Chidel M, Macklis RM. The role of radiation therapy in the management of Wilms' tumor. Semin Urol Oncol. 1999;17(1):46–54. [PubMed] [Google Scholar]
- 18.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms tumor. A report from the National Wilms Tumor Study Group. J Clin Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 19.Evans AE, Norkool P, Evans I, et al. Late effects of treatment for Wilms' tumor. Cancer. 1991;67:331–338. doi: 10.1002/1097-0142(19910115)67:2<331::aid-cncr2820670202>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Breslow NE, Takashima JR, Whitton JA, et al. Second malignant neoplasms following treatment for Wilms tumor - A report from the National Wilms Tumor Study Group. J Clin Oncol. 1995;13(8):1851–1859. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 21.Green DM, Peabody EM, Nan B, et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20(10):2506–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 22.Breslow NE, Ou SS, Beckwith JB, et al. Doxorubicin for favorable histology, Stage II-III Wilms tumor - Results from the National Wilms Tumor Studies. Cancer. 2004;101(5):1072–1080. doi: 10.1002/cncr.20433. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:456–481. [Google Scholar]
- 24.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Roy Stat Soc A. 1972;135(2):185–206. [Google Scholar]
- 25.Cox DR. Regression models and life-tables (with discussion) J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 26.Beckwith JB. National Wilms Tumor Study: An update for pathologists. Pediatr Dev Pathol. 1998;1:79–84. doi: 10.1007/s100249900010. [DOI] [PubMed] [Google Scholar]
- 27.Grundy P, Breslow N, Green DM, et al. Prognostic factors for children with recurrent Wilms' tumor: Results from the second and third National Wilms' Tumor Study. J Clin Oncol. 1989;7(5):638–647. doi: 10.1200/JCO.1989.7.5.638. [DOI] [PubMed] [Google Scholar]
- 28.Beckwith JB, Zuppan CE, Browning NG, et al. Histological analysis of aggressiveness and responsiveness in Wilms' tumor. Med Pediatr Oncol. 1996;27(5):422–428. doi: 10.1002/(SICI)1096-911X(199611)27:5<422::AID-MPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]


