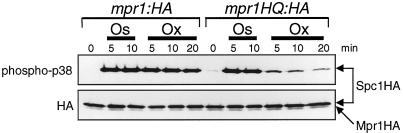
Figure 3.
The putative histidine phosphorylation site, His-221, is required for Mpr1 function in oxidative stress signaling to Spc1. The mpr1HQ mutant gene with the His-221→Gln substitution was created by site-directed mutagenesis and fused to the HA6H tag, which was used to replace the mpr1+ locus of an spc1:HA6H strain. The resultant strain (CA403) and the control strain, which has the wild-type mpr1+ locus tagged with HA6H (CA385), were grown to midlog phase at 30°C in YES medium and then treated with osmostress induced by 0.6 M KCl (Os) and oxidative stress induced by 0.3 mM H2O2 (Ox) for the indicated times. Spc1 as well as wild-type and mutant Mpr1 were purified on Ni-NTA beads and analyzed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. The Mpr1 and Mpr1HQ proteins were detected as weak bands below the Spc1 bands in anti-HA immunoblotting. Stress-induced Spc1 activation in the mpr1:HA strain was indistinguishable from that in strains expressing untagged Mpr1, indicating that the HA6H tag does not disturb the Mpr1 function.