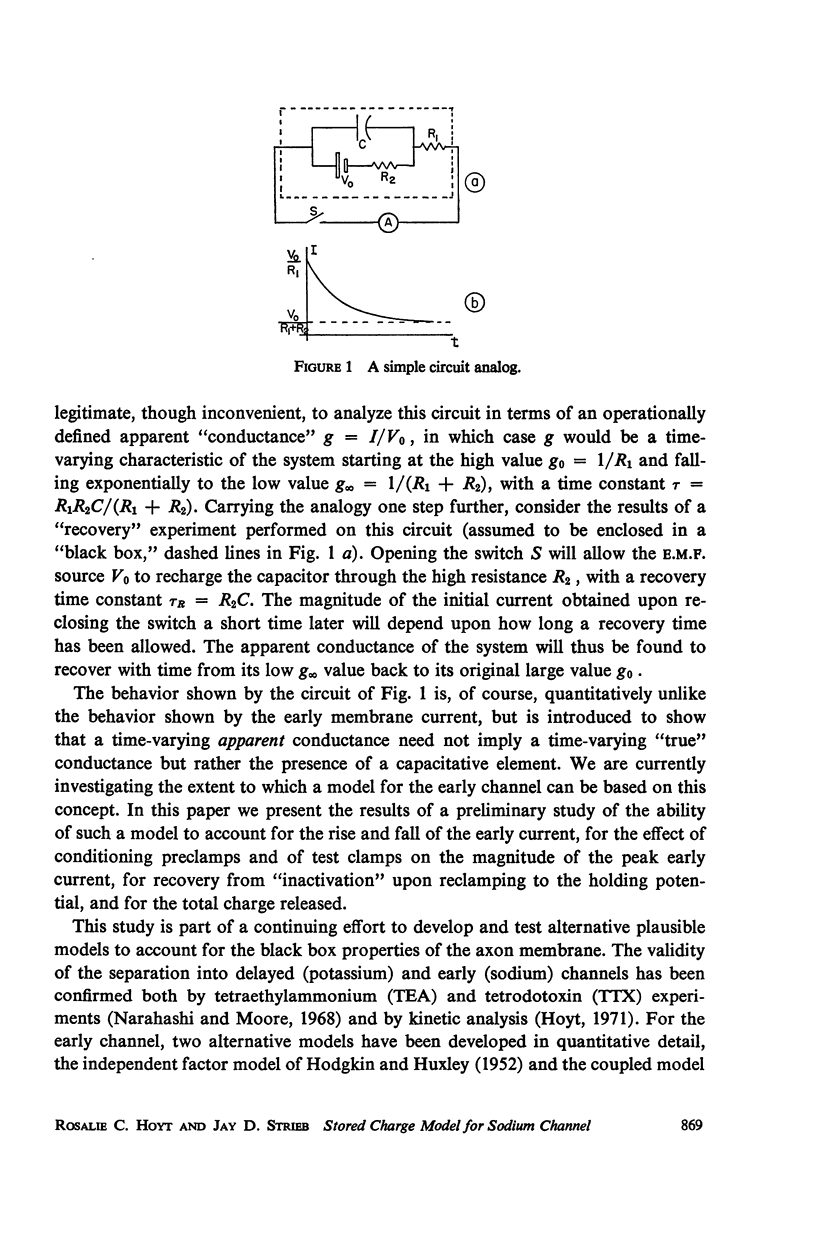
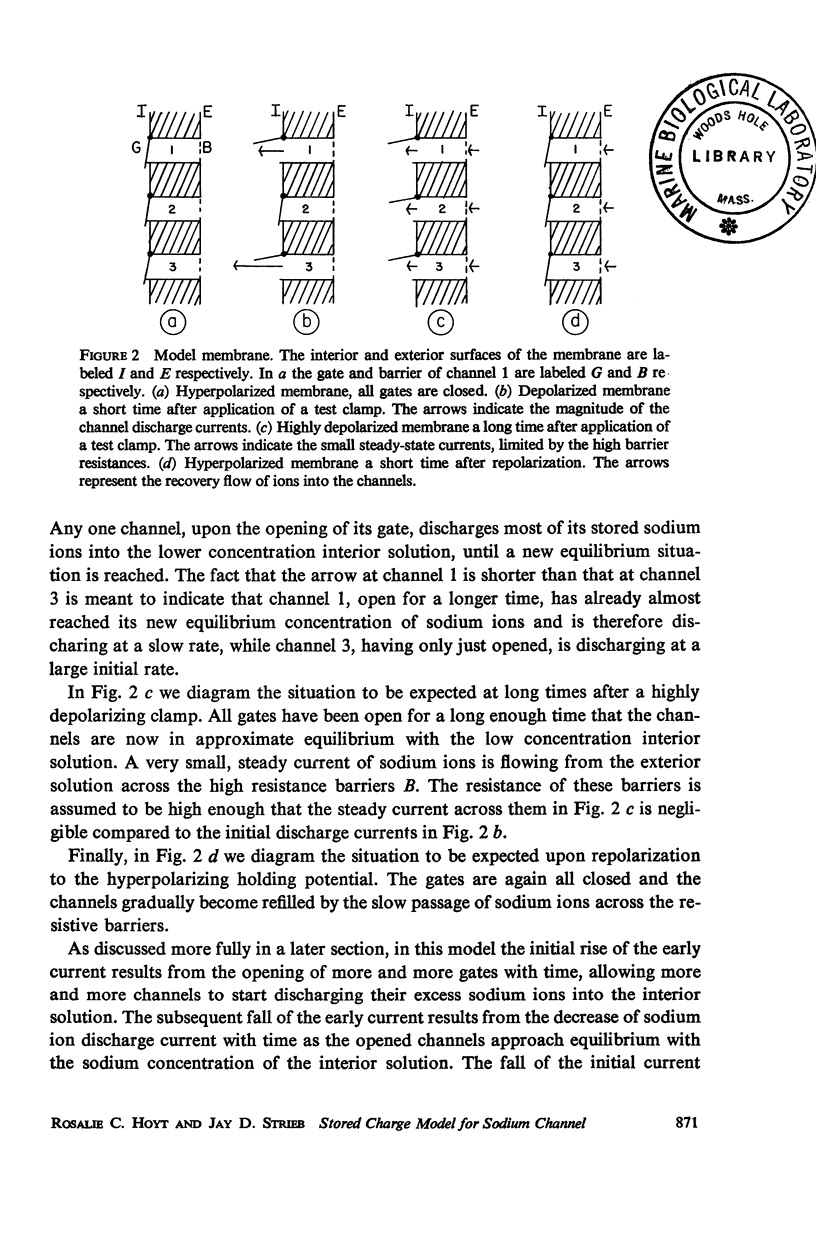
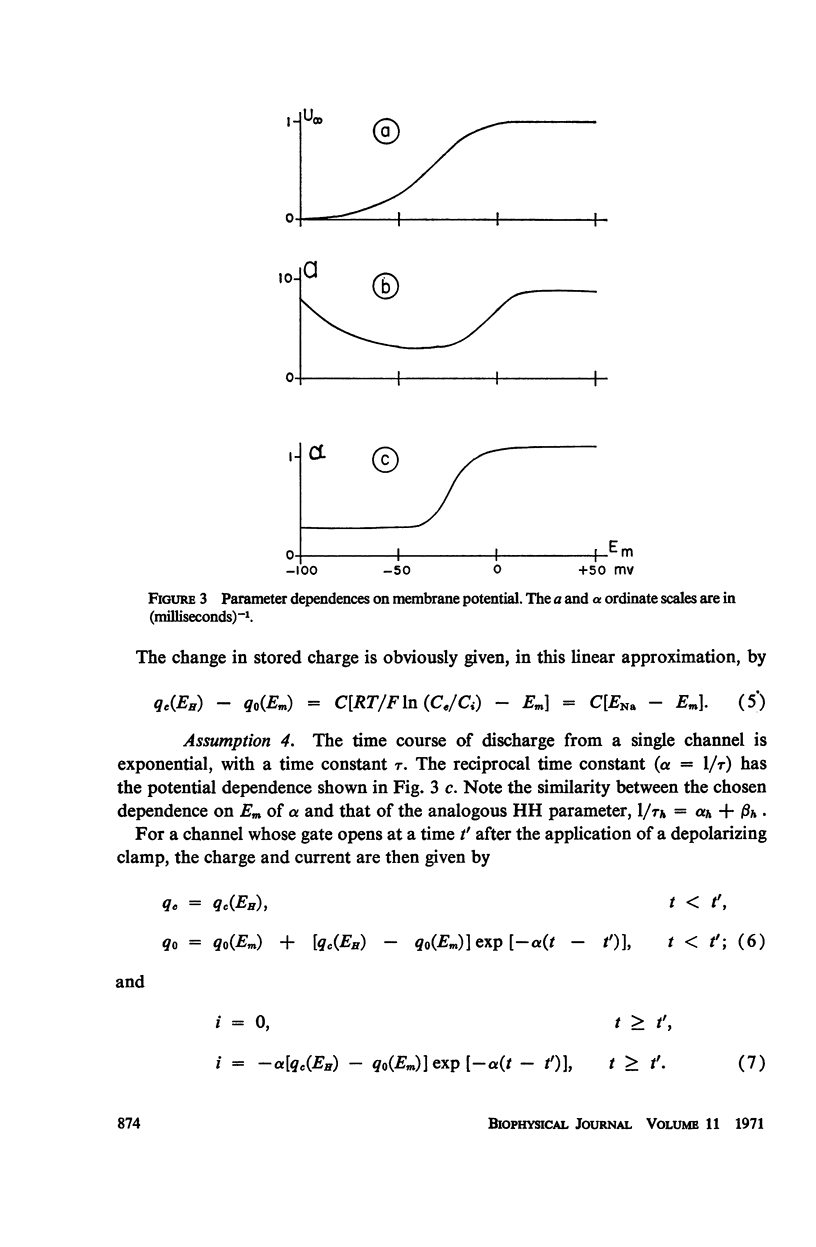
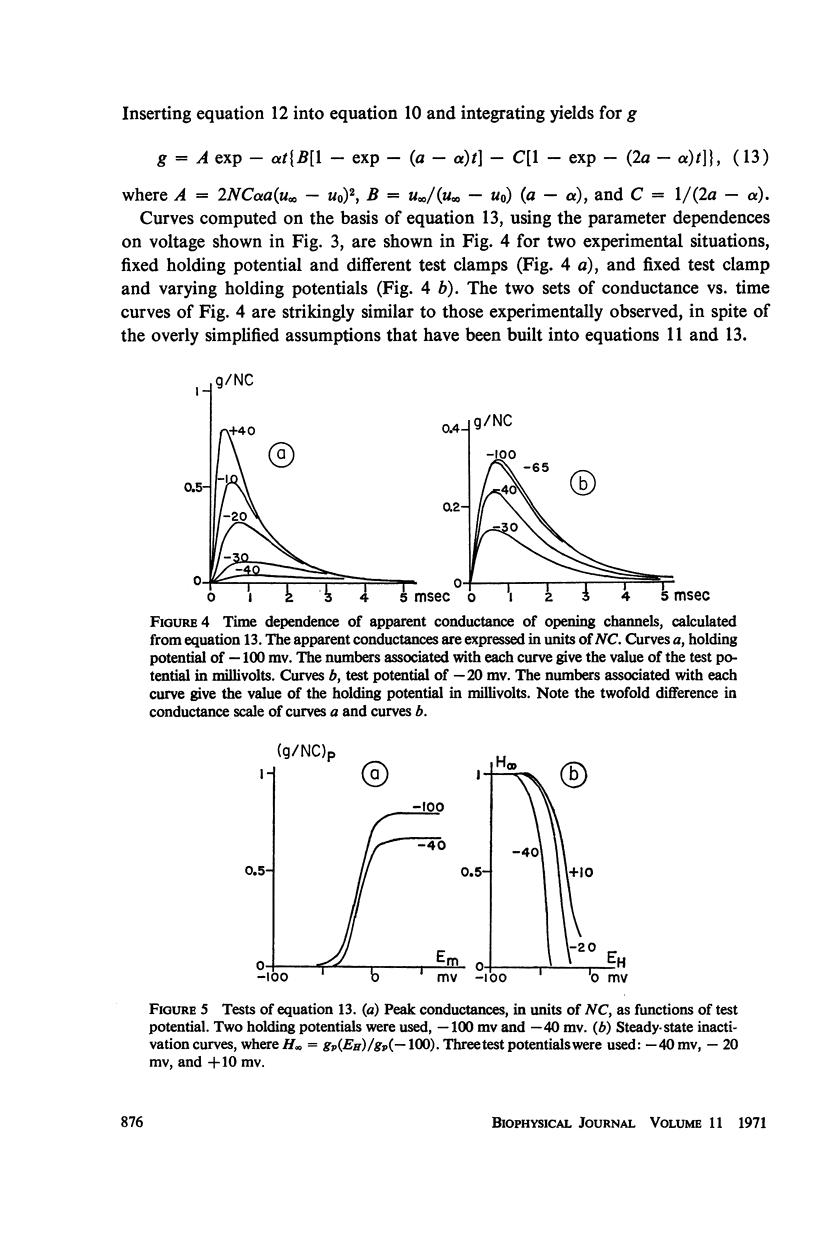
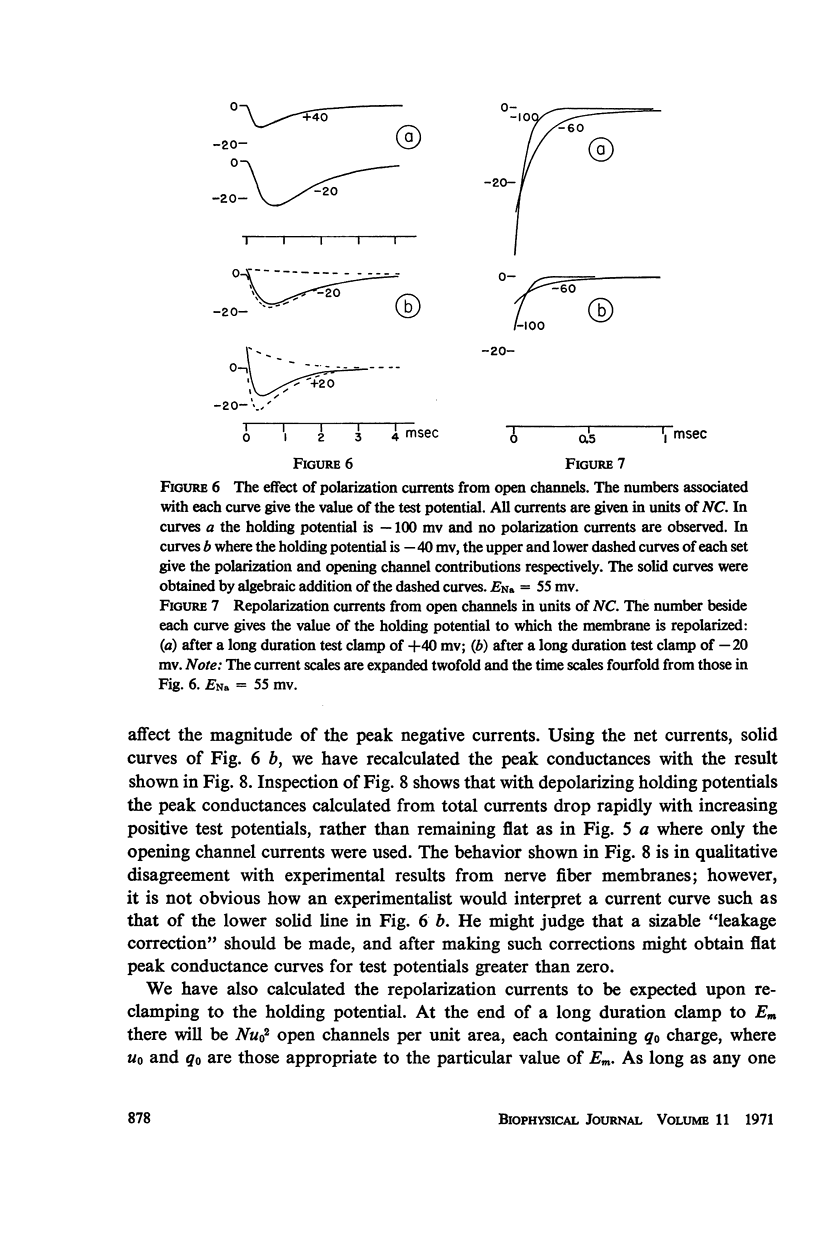
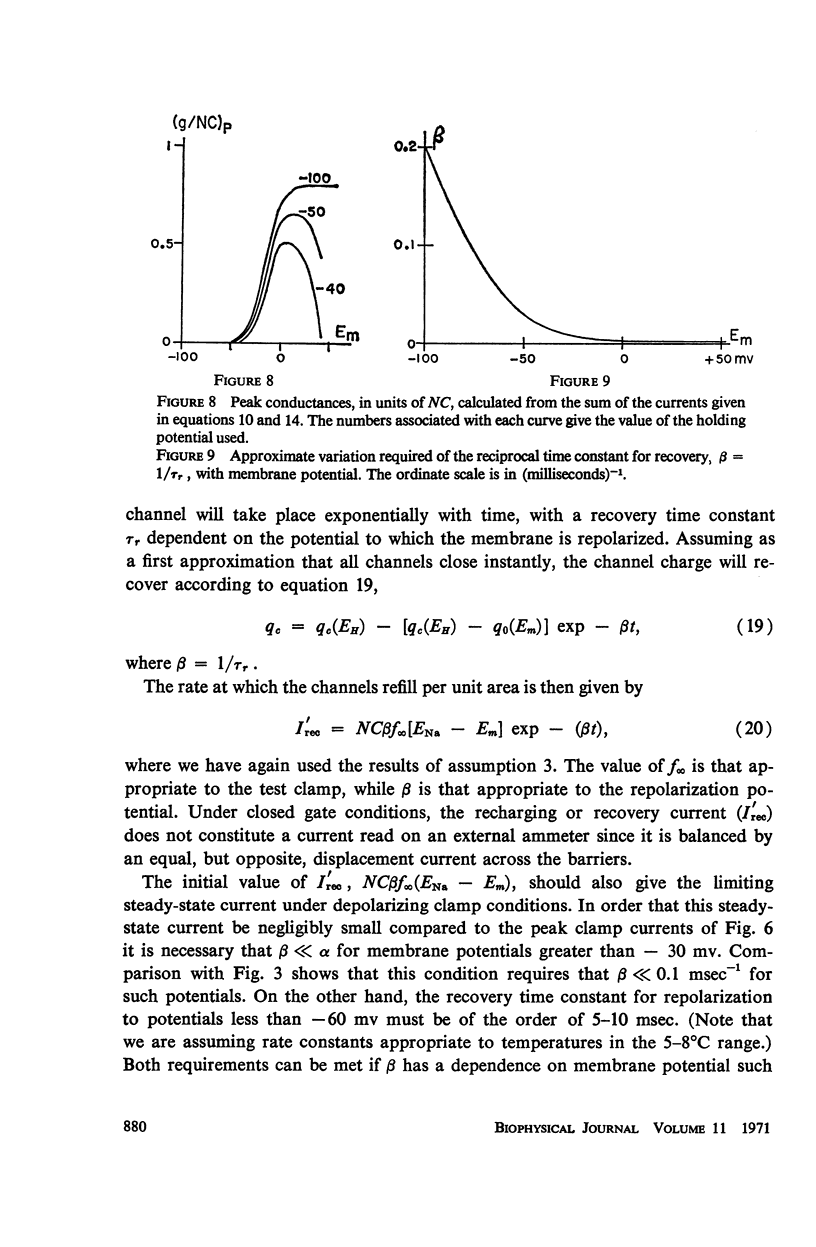
Abstract
A new model is proposed to account for the apparent conductance changes of the sodium, or early, channel in nerve fiber membranes. In this model it is assumed that the channels are gated at the interior side of the membrane and are resistively limited at the exterior side by sodium selective barriers of high resistance to ion flow. Under resting conditions the closed channels accumulate a store of sodium ions, dependent on the exterior sodium concentration. With the application of a depolarizing clamp the interior gates open allowing the stored ions to discharge into the interior low sodium concentration solution. In this model the initial rise in the early current results from the opening of more and more gates in response to the depolarizing clamp. The subsequent fall in the early current results from the “capacitative” discharge of the opened channels, limited by the high resistive barrier at the exterior end. Upon repolarization, the gates reclose and sodium ions reaccumulate in the channels from the high concentration external solution, but at a slow rate determined by the resistive barrier. Preliminary tests of this model, using a number of simplifying assumptions, show that it has the ability to account, at least semiquantitatively, for the major characteristics of the experimental clamp results.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C., Adelman W. J., Jr Sodium inactivation. Experimental test of two models. Biophys J. 1970 Jul;10(7):610–617. doi: 10.1016/S0006-3495(70)86323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C. Independence of the sodium and potassium conductance channels. A kinetic argument. Biophys J. 1971 Jan;11(1):110–122. doi: 10.1016/S0006-3495(71)86199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C. Sodium inactivation in nerve fibers. Biophys J. 1968 Oct;8(10):1074–1097. doi: 10.1016/S0006-3495(68)86540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]


