Abstract
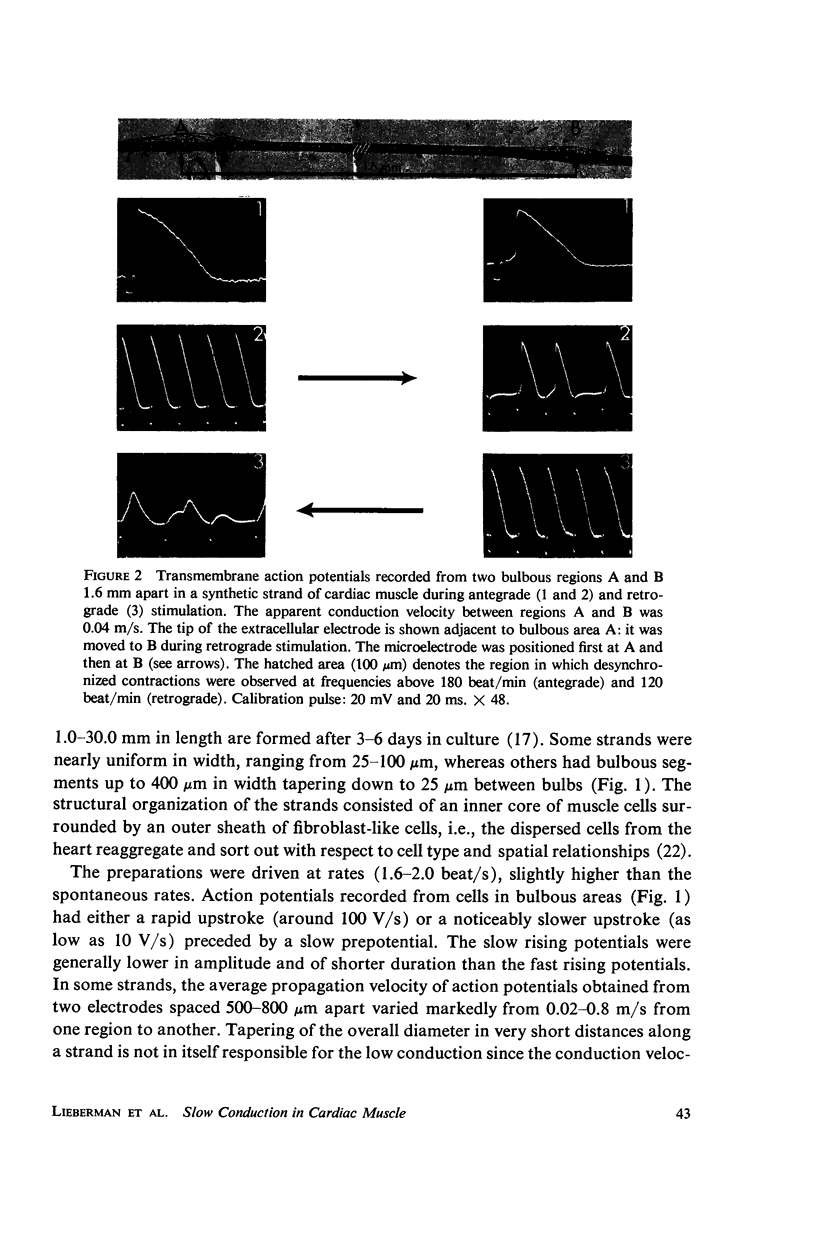
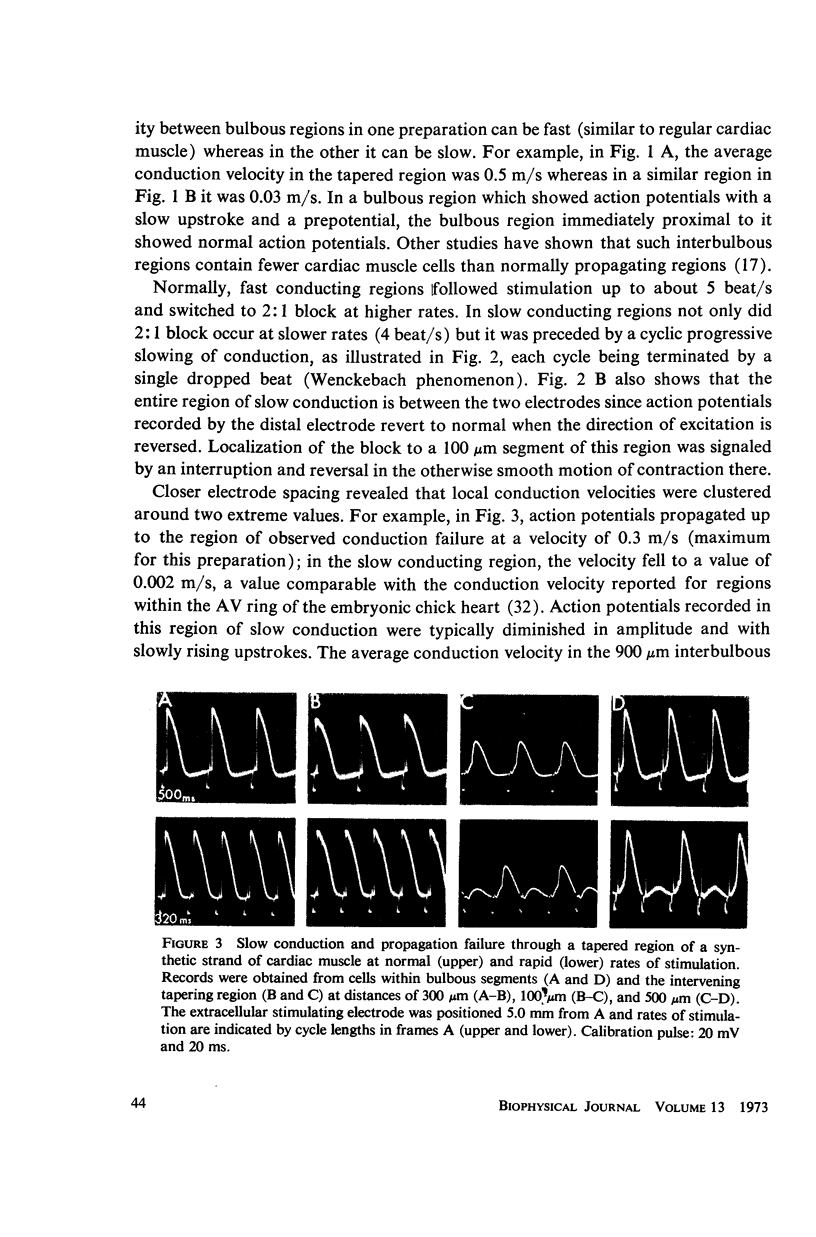
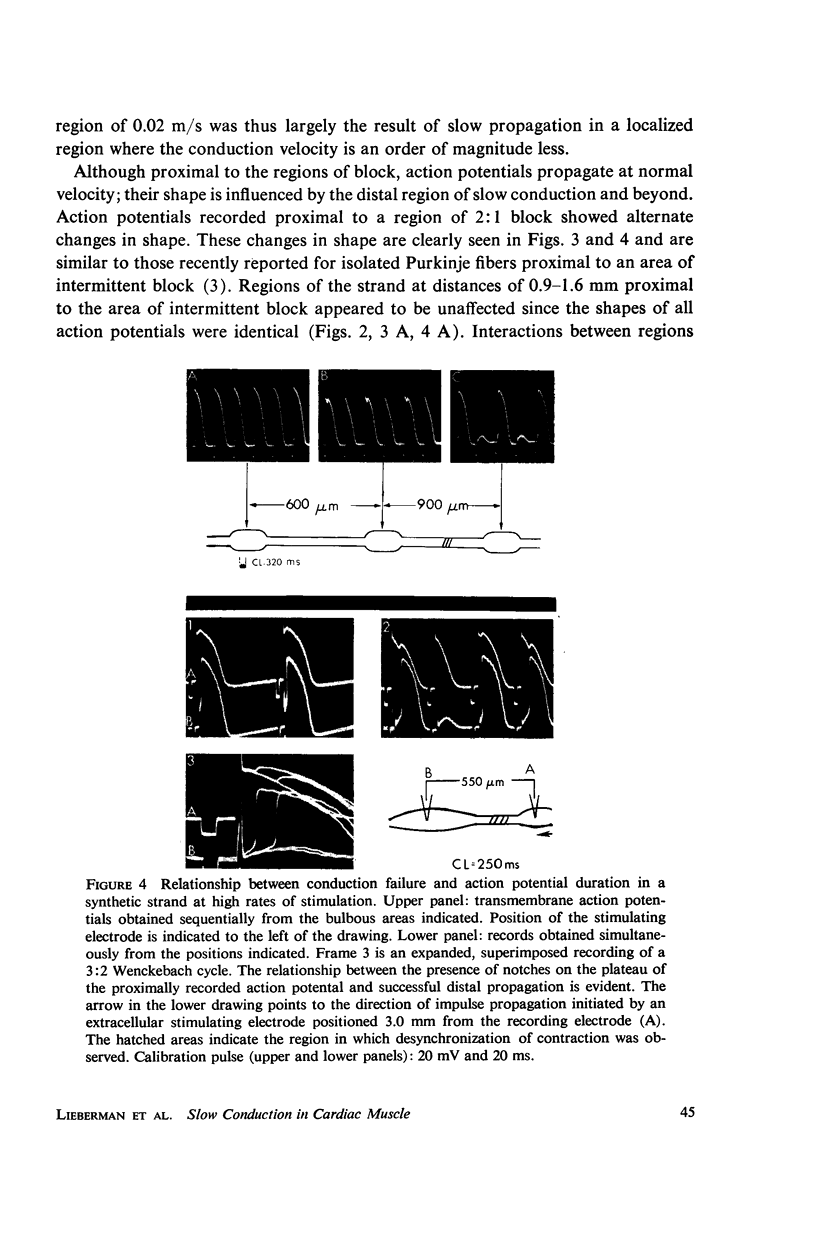
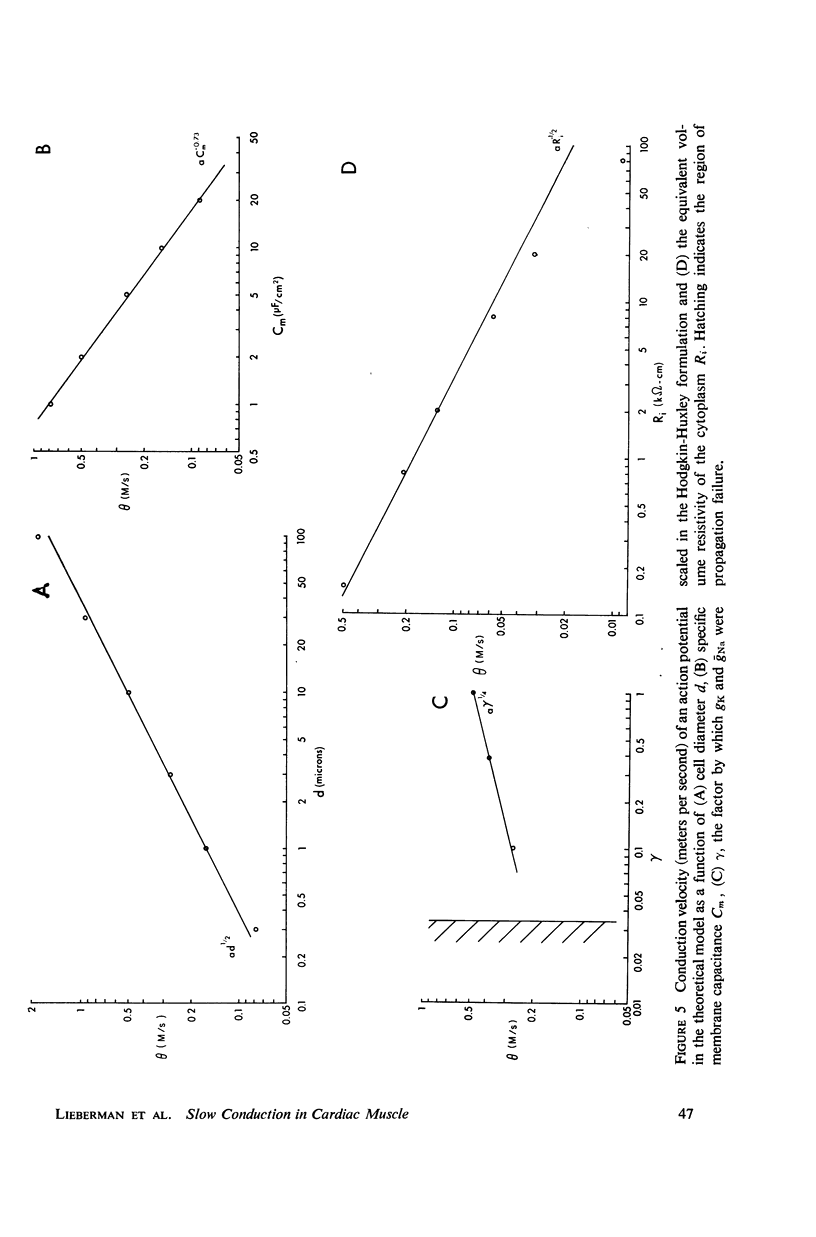
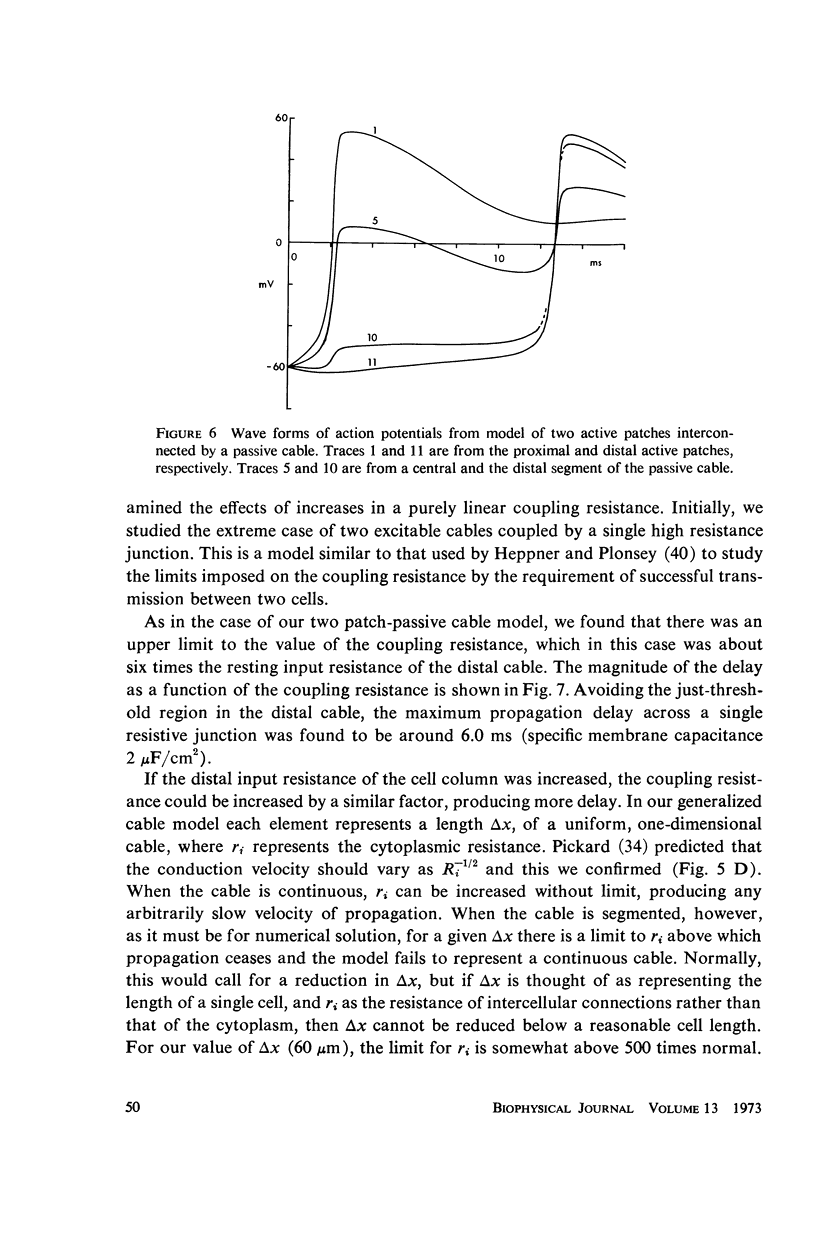
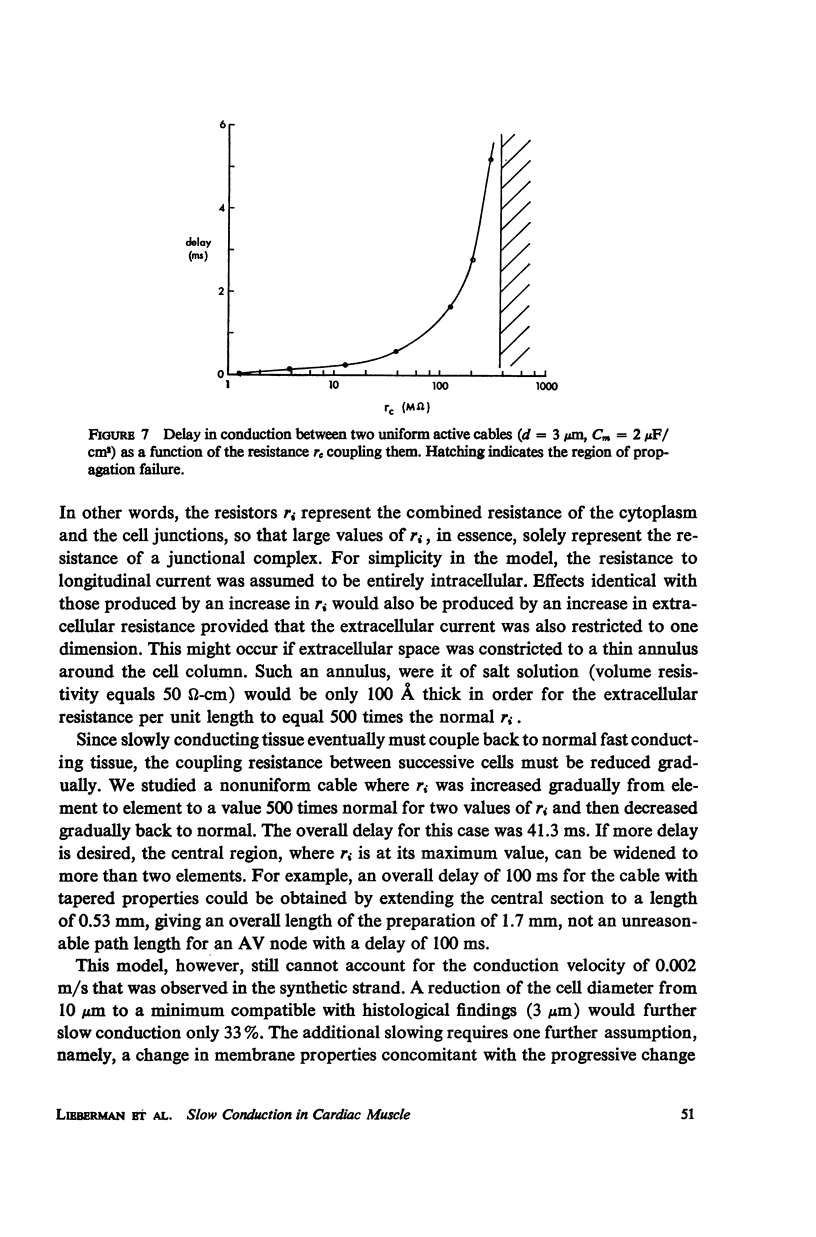
Mechanisms of slow conduction in cardiac muscle are categorized and the most likely identified. Propagating action potentials were obtained experimentally from a synthetically grown strand of cardiac muscle (around 50 μm by 30 mm) and theoretically from a one-dimensional cable model that incorporated varying axial resistance and membrane properties along its length. Action potentials propagated at about 0.3 m/s, but in some synthetic strands there were regions (approximately 100 μm in length) where the velocity decreased to 0.002 m/s. The electrophysiological behavior associated with this slow conduction was similar to that associated with slow conduction in naturally occurring cardiac muscle (notches, Wenckebach phenomena, and block). Theoretically, reasonable changes in specific membrane capacitance, membrane activity, and various changes in geometry were insufficient to account for the observed slow conduction velocities. Conduction velocities as low as 0.009 m/s, however, could be obtained by increasing the resistance (ri) of connections between the cells in the cable; velocities as low as 0.0005 m/s could be obtained by a further increase in ri made possible by a reduction in membrane activity by one-fourth, which in itself decreased conduction velocity by only a factor of 1/1.4. As a result of these findings, several of the mechanisms that have been postulated, previously, are shown to be incapable of accounting for delays such as those which occur in the synthetic strand as well as in the atrioventricular (VA) node.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALANIS J., BENITEZ D., PILAR G. A functional discontinuity between the Purkinje and ventricular muscle cells. Acta Physiol Lat Am. 1961;11:171–183. [PubMed] [Google Scholar]
- BARR L., DEWEY M. M., BERGER W. PROPAGATION OF ACTION POTENTIALS AND THE STRUCTURE OF THE NEXUS IN CARDIAC MUSCLE. J Gen Physiol. 1965 May;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K. M. The fine structure and electrophysiology of heart muscle cell injury. J Cell Biol. 1970 Sep;46(3):455–476. doi: 10.1083/jcb.46.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Noble S. J. Membrane currents underlying delayed rectification and pace-maker activity in frog atrial muscle. J Physiol. 1969 Oct;204(3):717–736. doi: 10.1113/jphysiol.1969.sp008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranefield P. F., Klein H. O., Hoffman B. F. Conduction of the cardiac impulse. 1. Delay, block, and one-way block in depressed Purkinje fibers. Circ Res. 1971 Feb;28(2):199–219. doi: 10.1161/01.res.28.2.199. [DOI] [PubMed] [Google Scholar]
- Cranefield P. F., Wit A. L., Hoffman B. F. Conduction of the cardiac impulse. 3. Characteristics of very slow conduction. J Gen Physiol. 1972 Feb;59(2):227–246. doi: 10.1085/jgp.59.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWEY M. M., BARR L. A STUDY OF THE STRUCTURE AND DISTRIBUTION OF THE NEXUS. J Cell Biol. 1964 Dec;23:553–585. doi: 10.1083/jcb.23.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice L. J., Challice C. E. Anatomical and ultrastructural study of the electrophysiological atrioventricular node of the rabbit. Circ Res. 1969 Mar;24(3):457–474. doi: 10.1161/01.res.24.3.457. [DOI] [PubMed] [Google Scholar]
- FITZHUGH R. Thresholds and plateaus in the Hodgkin-Huxley nerve equations. J Gen Physiol. 1960 May;43:867–896. doi: 10.1085/jgp.43.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay W. A., Jr, Johnson E. A. An anatomical evaluation of the myocardial length-tension diagram. Circ Res. 1967 Jul;21(1):33–43. doi: 10.1161/01.res.21.1.33. [DOI] [PubMed] [Google Scholar]
- Goshima K. Synchronized beating of and electrotonic transmission between myocardial cells mediated by heterotypic strain cells in monolayer culture. Exp Cell Res. 1969 Dec;58(2):420–426. doi: 10.1016/0014-4827(69)90523-0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L. A note on conduction velocity. J Physiol. 1954 Jul 28;125(1):221–224. doi: 10.1113/jphysiol.1954.sp005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth O. Effects of halothane on single atrial, ventricular, and Purkinje fibers. Circ Res. 1969 May;24(5):745–750. doi: 10.1161/01.res.24.5.745. [DOI] [PubMed] [Google Scholar]
- Heppner D. B., Plonsey R. Simulation of electrical interaction of cardiac cells. Biophys J. 1970 Nov;10(11):1057–1075. doi: 10.1016/S0006-3495(70)86352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde A., Blondel B., Matter A., Cheneval J. P., Filloux B., Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- James T. N., Sherf L. Ultrastructure of the human atrioventricular node. Circulation. 1968 Jun;37(6):1049–1070. doi: 10.1161/01.cir.37.6.1049. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kanno T. The heterogenous structure of the specialized tissue in the heart as a factor in atrioventricular conduction delay. Jpn J Physiol. 1970 Aug;20(4):417–434. doi: 10.2170/jjphysiol.20.417. [DOI] [PubMed] [Google Scholar]
- Lieberman M. Effects of cell density and low K on action potentials of cultured chick heart cells. Circ Res. 1967 Dec;21(6):879–888. doi: 10.1161/01.res.21.6.879. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Paes de Carvalho A. The Spread of Excitation in the Embryonic Chick Heart. J Gen Physiol. 1965 Nov 1;49(2):365–379. doi: 10.1085/jgp.49.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Roggeveen A. E., Purdy J. E., Johnson E. A. Synthetic strands of cardiac muscle: growth and physiological implication. Science. 1972 Feb 25;175(4024):909–911. doi: 10.1126/science.175.4024.909. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Alanis J., Benitez D. Transitional cardiac cells of the conductive system of the dog heart. Distinguishing morphological and electrophysiological features. J Cell Biol. 1970 Oct;47(1):1–17. doi: 10.1083/jcb.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez C., Mueller W. J., Urguiaga X. Propagation of impulses across the Prukinje fiber-muscle junctions in the dog heart. Circ Res. 1970 Feb;26(2):135–150. doi: 10.1161/01.res.26.2.135. [DOI] [PubMed] [Google Scholar]
- Merideth J., Mendez C., Mueller W. J., Moe G. K. Electrical excitability of atrioventricular nodal cells. Circ Res. 1968 Jul;23(1):69–85. doi: 10.1161/01.res.23.1.69. [DOI] [PubMed] [Google Scholar]
- NOBLE D. A modification of the Hodgkin--Huxley equations applicable to Purkinje fibre action and pace-maker potentials. J Physiol. 1962 Feb;160:317–352. doi: 10.1113/jphysiol.1962.sp006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard W. F. On the propagation of the nervous impulse down medullated and unmedullated fibers. J Theor Biol. 1966 May;11(1):30–45. doi: 10.1016/0022-5193(66)90036-1. [DOI] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- SANO T., SUZUKI F., TAKIGAWA S. THE GENESIS OF THE STEP OR NOTCH ON THE UPSTROKE OF THE ACTION POTENTIAL OBTAINED FROM THE ATRIO-VENTRICULAR NODE. Jpn J Physiol. 1964 Dec 15;14:659–668. doi: 10.2170/jjphysiol.14.659. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Lieberman M., Scott J. G., Barr R. C., Johnson E. A., Kootsey J. M. Excitation sequences of the atrial septum and the AV node in isolated hearts of the dog and rabbit. Circ Res. 1971 Aug;29(2):156–172. doi: 10.1161/01.res.29.2.156. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Mayer G., Macdonald R. Velocity of propagation in vertebrate cardiac muscles as functions of tonicity and [K+]. Am J Physiol. 1970 Oct;219(4):952–963. doi: 10.1152/ajplegacy.1970.219.4.952. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Bennett C. M., Jamison R. L., Troy J. L. An air-driven micropipette sharpener. J Appl Physiol. 1967 Jan;22(1):191–192. doi: 10.1152/jappl.1967.22.1.191. [DOI] [PubMed] [Google Scholar]
- Wennemark J. R., Ruesta V. J., Brody D. A. Microelectrode study of delayed conduction in the canine right bundle branch. Circ Res. 1968 Dec;23(6):753–769. doi: 10.1161/01.res.23.6.753. [DOI] [PubMed] [Google Scholar]
- de Carvalho A. P., Hoffman B. F., de Carvalho M. P. Two components of the cardiac action potential. I. Voltage-time course and the effect of acetylcholine on atrial and nodal cells of the rabbit heart. J Gen Physiol. 1969 Nov;54(5):607–635. doi: 10.1085/jgp.54.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]