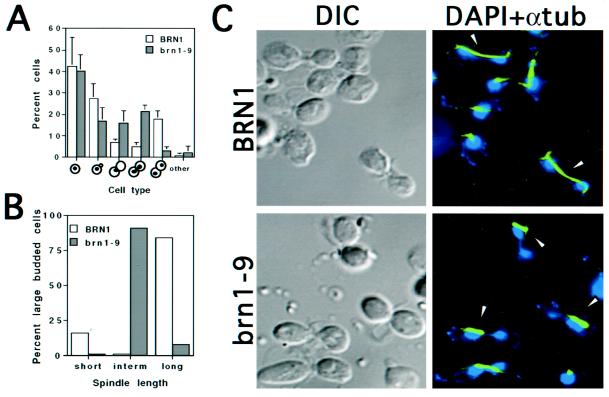
Figure 1.
Phenotypic characteristics of brn1 mutants at the restrictive temperature. (A) Distribution of cell types. Asynchronous cultures of strains CH2523 (BRN1) and CH2524 (brn1-9) were shifted to 37°C for 3 h, fixed, and stained with DAPI to visualize nuclei. Cells were scored according to bud morphology and position/distribution of nuclear DNA, as indicated. (B) Spindle length in large-budded BRN1 and brn1-9 cells. Large-budded cells from strains grown as described for A were examined for spindle length. Short spindles refers to the 1- to 2-μm length typical of a metaphase cell, intermediate spindles are 4–5 μm in length, and long spindles range from 7 to 15 μm, typical of anaphase/telophase cells. At least 100 large-budded cells were scored per sample. (C) Nuclear and spindle morphology. Cells were grown as described for A, fixed, prepared for indirect immunofluorescence of tubulin (see MATERIALS AND METHODS), and stained with DAPI to visualize DNA. Micrographs were false colored in blue (DNA) and green (anti-tubulin). Arrowheads denote extended spindles in dividing BRN1 (wild-type) cells in contrast to the thick, intermediate spindles found in the large-budded brn1-9 cells. Note that in the mutant, the spindle is often associated with only one side of the bilobed DNA mass and/or grossly mispositioned (see DISCUSSION). In addition, the bilobed nuclei present in BRN1 cells are morphologically distinct from those in the mutant; in general, the BRN1 cells show very little chromosomal material joining two equal DNA masses, suggesting the rapid progression of anaphase to telophase in BRN1 cells. In the brn1 mutant, however, anaphase appears to initiate normally but proceeds with difficulty, yielding cells with two connected chromatin masses. In these early to mid anaphase cells, >40% of bilobed nuclear masses showed uneven DAPI staining. DIC, differential interference contrast microscopy.