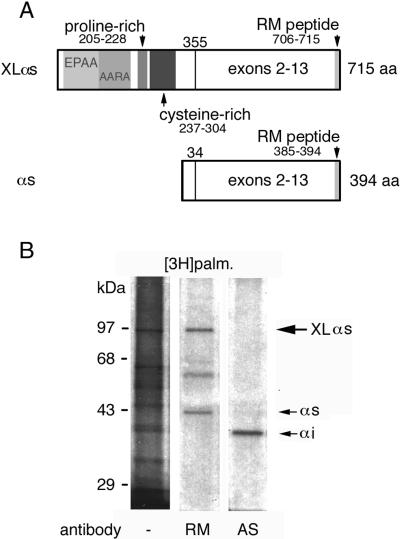
Figure 1.
(A) Domain organization of XLαs. XLαs is identical to Gαs with respect to exons 2–13. The alternatively spliced amino terminus contains areas with EPAA repeats (residues 37–104), ARAA repeats (residues 103–187), and the proline-rich (residues 205–228) and cysteine-rich (residues 237–304) domains. The RM antibody, which recognizes both XLαs and Gαs, was generated from the decapeptide sequence at the carboxy termini of these proteins. (B) Incorporation of [3H]palmitate into endogenous XLαs. PC-12 cells were incubated with [3H]palmitate, harvested, and separated into particulate and soluble fractions. One milligram of protein from the particulate fractions underwent immunoprecipitation with either the RM antibody or the AS antibody specific for Gαi. The immunoprecipitates and 20 μg of particulate fraction protein (lane 1) were analyzed by SDS-PAGE and indirect fluorography. Fluorographs were exposed for 1 mo at −70°C.