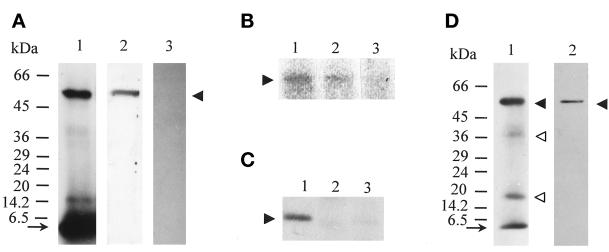
Figure 5.
The 55-kDa protein, copurified with p15, is recognized by anti-GA/1 antibodies, is ADP-ribosylated by cholera toxin, and binds GTP. (A) Affinity material was either radioiodinated and analyzed by electrophoresis and autoradiography as in Figure 3 (lane 1) or analyzed by immunoblotting with anti-GA/1 antibodies in the absence and presence of peptide GA/1 (40 μM; lanes 2 and 3, respectively). Recognition of the 55-kDa protein is indicated by a filled arrowhead. (B) Affinity material was incubated either with activated cholera toxin and [32P]NAD, in the absence and presence of GTPγS (0.1 mM; lanes 1 and 2, respectively), or only with [32P]NAD (lane 3), fractionated on SDS-PAGE, and gels were scanned for radioactivity with a molecular imager system. The radiolabeled 55-kDa protein is indicated by a filled arrowhead. (C) Material was either eluted with GTP (0.1 mM in the presence of 0.5 mM MgCl2) from affinity chromatography loaded with membrane preparations previously not incubated or incubated with GTPγS (0.1 mM; lanes 1 and 2, respectively) or with ATP (0.1 mM in the presence of 0.5 mM MgCl2) from affinity chromatography loaded with membrane preparations not incubated with GTPγS (lane 3), and then analyzed by immunoblotting with anti-GA/1 antibodies. The 55-kDa protein detected in lane 1, and not detected in the two other lanes, is indicated by a filled arrowhead. (D) Material was eluted with [α-32P]GTP (16 nM, 3000 Ci/mmol) from affinity chromatography, exposed to UV irradiation, fractionated on SDS-PAGE, and blotted onto polyvinylidene difluoride filters. Blotted filters were then exposed to autoradiography (lane 1) or incubated with anti-GA/1 antibodies (lane 2). Complexes between [α-32P]GTP and the 55-kDa protein are indicated by filled arrowheads; two other minor species of radiolabeled complexes, not recognized by anti-GA/1 antibodies, are indicated by open arrowheads.