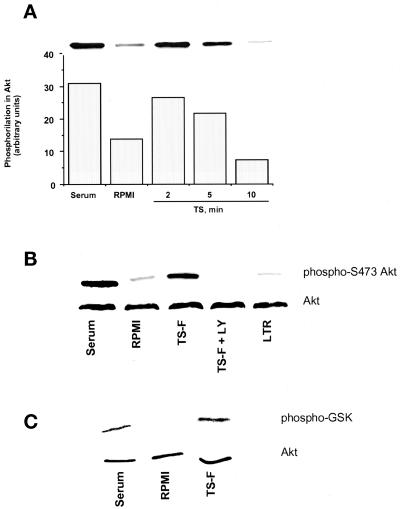
Figure 7.
Activation of signaling pathways in PC12 cells by TS. (A) TS induces serine phosphorylation of Akt kinase. PC12 cells were kept in RPMI containing 0.1% FCS for 2 d. Then some monolayers were switched to 20% FCS (Serum) and others to RPMI without (RPMI) and with 100 ng/ml TS (TS) for the indicated times. Cells were lysed in 2% SDS, and proteins in the lysate were resolved by SDS-PAGE on 10% gels and stained with anti-phospho-Akt antibody. The bar graph represents the quantitation by scanning densitometry of the corresponding bands in the immunoblot (inset). (B) TS-induced phosphorylation of Akt kinase is inhibited by the PI-3 kinase inhibitor LY294002. The protocol was similar to the one in A, except that, where indicated, cells were preincubated with 1 μM LY294002 (TS-F + LY) before the addition of 100 ng/ml catalytic domain of TS (TS-F). Note that LY294002 completely blocked TS-F-induced phosphorylation of Akt kinase. LY294002 was similarly effective in inhibiting Akt phosphorylation induced by full-length TS (our unpublished data). (C) TS-F induces PKB/Akt kinase activity. PC12 cells were treated as described in A. Then some were switched to 20% FCS (Serum) and others to RPMI without (RPMI) and with 100 ng/ml TS-F (TS-F) for 2 min. Cell lysates were immunoprecipitated with Akt antibodies coupled to agarose beads The resulting immunoprecipitates were incubated with GSK-3 fusion protein as an Akt kinase substrate. Phosphorylation of GSK-3 was measured by Western blotting using a phospho-GSK-3α/β (Ser-21/9) antibody.