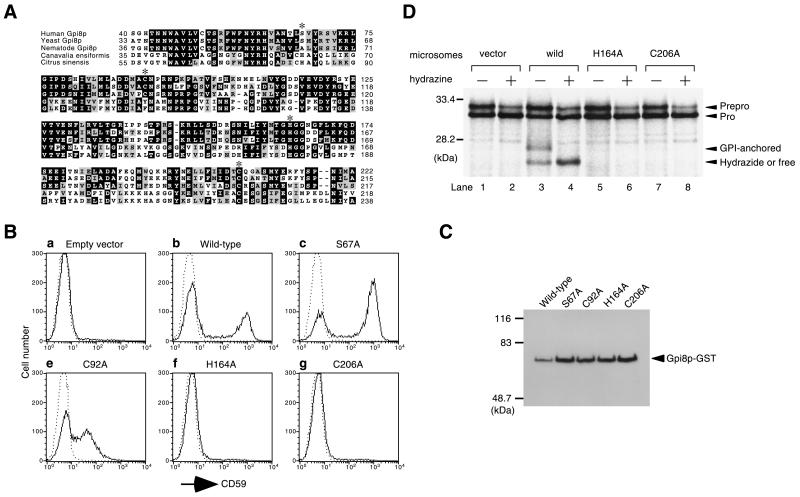
Figure 4.
Cysteine and histidine residues conserved in Gpi8ps and cysteine proteases are essential for Gpi8p activity. (A) Alignment of partial sequences of human Gpi8p, yeast Gpi8p, nematode (Caenorhabditis elegans) Gpi8p (T05E11.6) (Wilson et al., 1994), and two proteases belonging to a novel cysteine protease family from Canavalia ensiformis (Abe et al., 1993), Citrus sinensis (Alonso and Granell, 1995) using Clustal W software. Amino acids of human Gpi8p substituted for alanine are marked with asterisks. (B) Activities of alanine mutants of human Gpi8p. GST-tagged wild-type and mutant human GPI8 cDNAs were transiently transfected into class K cells. Cells were stained for CD59 and analyzed by flow cytometry 2 d after transfection. Solid and dotted lines indicate staining with anti-CD59 antibody and that with secondary reagent alone, respectively. (C) Expression of mutant Gpi8ps in the transfectants used in B. GST-tagged Gpi8ps in detergent extracts of the transfectants were collected with glutathione beads and analyzed by Western blotting with anti-GST antibody. Molecular markers in kilodaltons are indicated on the left. (D) In vitro assay with mini-PLAP. Microsomal membranes of class K cells stably expressing GST-tagged wild-type or mutant human Gpi8p were tested for the formation of carbonyl intermediates of mini-PLAP in vitro in a similar way as in Figure 3. Molecular markers in kilodaltons are indicated on the left.