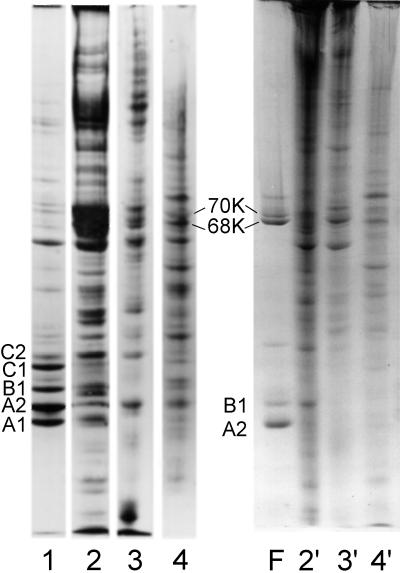
Figure 8.
Coomassie blue-stained SDS-PAGE showing the proteins present in a preparation of 40S hnRNP particles (lane 1) and rat liver, MEL cell, and HeLa cell nuclear matrix (lanes 2–4, respectively). The samples in lanes 1–4 were solubilized in standard SDS sample buffer containing mercaptoethanol. Matrix samples 2′, 3′, and 4′ are the same as those in lanes 2–4, but no reducing agent was present in the electrophoresis sample buffer. Lane F shows the proteins in an hnRNP filament preparation. The oxidized dimers of A2 and B1 are noted in lanes 4 and F. In the nonreduced samples (lanes 2′, 3′, and 4′) significant amounts of protein were trapped in the 3% stacking gel (our unpublished observations).