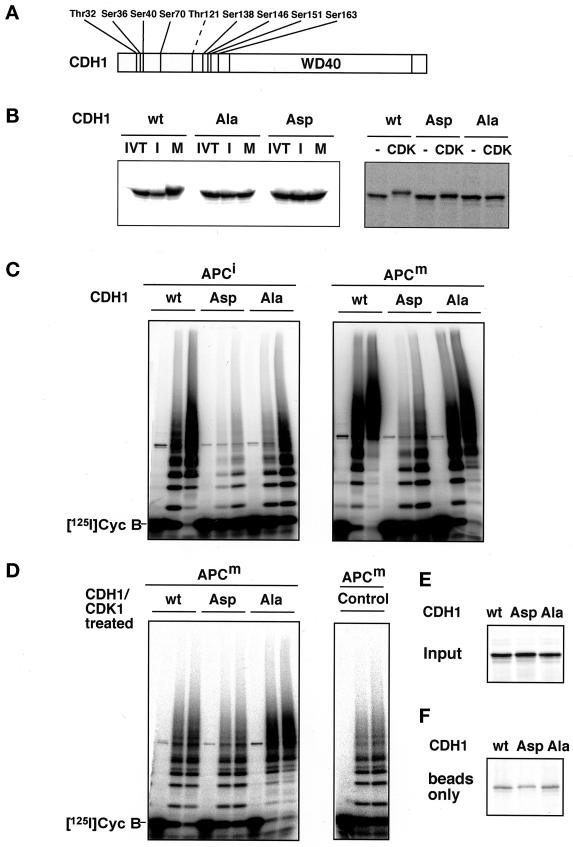
Figure 8.
Analysis of CDH1 phosphorylation mutants. (A) Schematic representation of the amino acid sequence of CDH1 and of the threonine and serine residues that were mutated to either alanine (all residues indicated; CDH1Ala) or aspartate (all residues indicated were mutated except Thr121 [dashed line]; CDH1Asp) residues is shown. The position of the WD40 repeat domain is indicated. (B) 35S-labeled in vitro translation products (IVT) of wild-type CDH1 (wt), CDH1Ala (Ala), and CDH1Asp (Asp) were incubated in interphase (I) or mitotic (M) Xenopus extracts (left) or in fractions from Sf9 cells infected with cyclin B/CDK1/p9 baculoviruses (CDK1) or not infected (−; right). All samples were subsequently analyzed by SDS-PAGE and phosphorimaging. (C) Immunopurified Xenopus interphase APC (APCi) or mitotic APC (APCm) were incubated with 35S-labeled proteins as described in B, washed stringently, and subsequently analyzed for cyclin B ubiquitination activity. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (D) In vitro–translated proteins as described in b were first incubated for 30 min in Sf9 cell fractions containing ectopically expressed cyclin B/CDK1/p9 complexes and then subjected to staurosporine treatment before they were added to immunopurified APC. In a control reaction (Control) mitotic APC was incubated with an in vitro translation mixture without DNA and analyzed as in C. After stringent washes, APC was analyzed for its cyclin B ubiquitination activity. (E) One-eightieth of the amount of 35S-labeled proteins used in C and D is shown. (F) The amount of 35S-labeled proteins that bind nonspecifically to the antibody-coupled beads is shown. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.