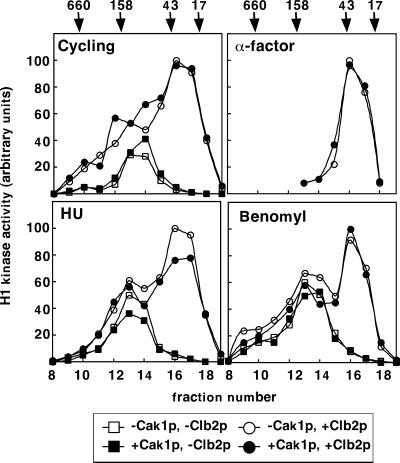
Figure 3.
Treatment with GST-Cak1p does not increase the H1 kinase activity of Cdc28p-HA from yeast extract. Cdc28p-HA was immunoprecipitated from fractions 13–18 of the α-factor–arrested extract and fractions 8–19 of the cycling, HU-arrested, and benomyl-arrested extracts (Figure 2A). Cdc28p-HA–containing beads were divided into four aliquots, incubated with buffer (□), GST-Cak1p only (▪), MBP-Clb2p only (○), or GST-Cak1p and MBP-Clb2p (●), and assayed for histone H1 kinase activity. Activity was quantitated with the use of a phosphorimager and plotted with the highest level of activity for each extract normalized to 100%. The activity of Cdc28p-HA from the α-factor–arrested extract was undetectable in the absence of cyclin. Gel filtration standards in kDa are as in Figure 2A.