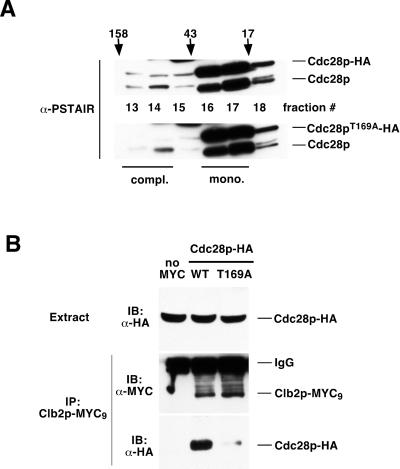
Figure 7.
Cdc28pT169A-HA is defective at binding cyclin in vivo. (A) Extracts were prepared from YKR102 (Cdc28p-HA; top) and YKR103 (Cdc28pT169A-HA; bottom) and gel filtered on a Superdex 200 column, and fractions 13–18 were blotted with the α-PSTAIR antibody. The faint band just above the untagged Cdc28p appears to be a degradation product of the HA-tagged Cdc28p. Note the decreased amount of Cdc28pT169A in fraction 14, indicating reduced cyclin binding. Gel filtration standards of 158, 43, and 17 kDa, and the peaks of Cdc28p-HA complexes (compl.) and monomer (mono.), are indicated. (B) Extracts were prepared from YKR108 [CDC28(HA) GAL-CLB2(MYC9); WT] and YKR109 [CDC28(HA)T169A GAL-CLB2(MYC9); T169A] grown in galactose to induce expression of Clb2p-MYC9 and from YKR107 grown in glucose to repress expression of the MYC-tagged protein (no MYC). Total extracts were immunoblotted (IB) with α-HA antibodies to detect Cdc28p-HA (top). Clb2p-MYC9 was immunoprecipitated (IP) with α-MYC antibodies, and the pellets were immunoblotted with α-MYC to detect Clb2p-MYC9 (middle) and with α-HA to detect coprecipitating Cdc28p-HA (bottom).