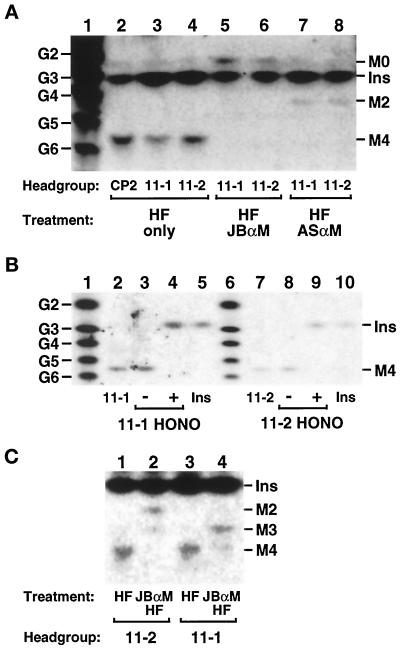
Figure 7.
Analysis of the glycan head groups of lipids 11-1 and 11-2. Lipids 11-1 and 11-2 were radiolabeled at 37°C with [3H]inositol in the gpi11:: LEU2-pPIG-F strain, and CP2 was radiolabeled at 37°C in gpi8 cells. The three lipids were isolated by preparative TLC and deacylated by treatment with mild base. (A) Size analysis and α-mannosidase sensitivity. Deacylated head groups were re-N-acetylated with acetic anhydride, then dephosphorylated with HF, and samples of them were treated with JBαM (lanes 5 and 6), with ASαM (lanes 7 and 8), or not treated (lanes 2–4). Glycans were then separated by HPTLC and detected by fluorography. Lane 1 displays a series of NaB[3H]4-reduced glucose2 (G2)-glucose6 (G6) oligomers, and the known mobilities of [3H]inositol-labeled GPI glycans and [3H]inositol are indicated. (B) Nitrous acid deamination. Deacylated, N-acetylated, and dephosphorylated glycans were submitted to nitrous acid (HONO) deamination (lanes 4 and 9) or mock treated (lanes 3 and 8). Lanes 2 and 7 of the HPTLC contain [3H]inositol-labeled material released from lipids 11-1 and 11-2 after mild base, acetic anhydride, and HF treatment; lanes 5 and 10 contain [3H]inositol; and lanes 1 and 6 contain NaB[3H]4-reduced glucose oligomers. (C) Positioning of HF-labile substituents. Deacylated, N-acetylated head groups were dephosphorylated directly with HF or treated first with JBαM and then with HF before separation by HPTLC. Traces of Man3 and Man4 glycans in lanes 2 and 4, respectively, most likely originate from incomplete JBαM digestion. The [3H]inositol present in glycan samples in A and C is a contaminant from the preparative TLC. Ins, M0, M2, M3, and M4, [3H]Ins, GlcNAc-[3H]Ins, Man2-GlcNAc-[3H]Ins, Man3-GlcNAc-[3H]Ins, and Man4-GlcNAc-[3H]Ins, respectively. HPTLC plates were exposed to x-ray film for 30 d (A), 10 d (B), and 21 d (C).