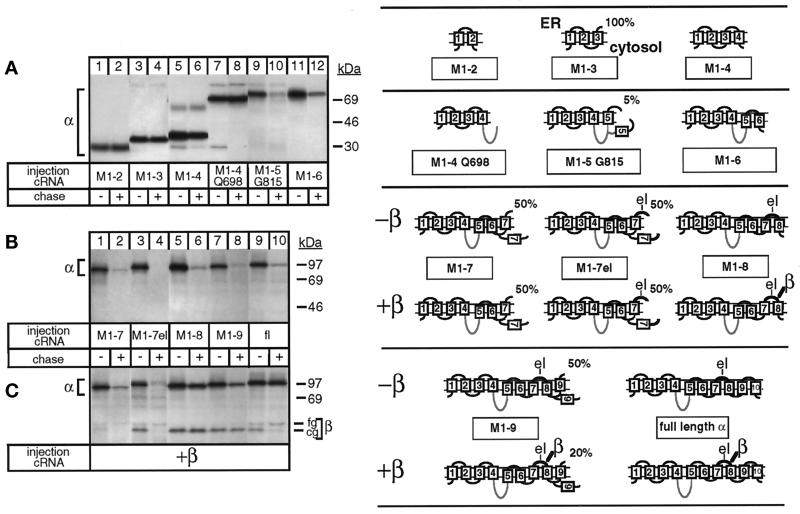
Figure 1.
Degradation of the unassembled Na,K-ATPase α subunit and its protection by β subunit assembly. Oocytes were injected with cRNA coding for truncated Na,K-ATPase α subunits (for description see Table 1) in the absence (A and B) or presence (C) of cRNA coding for Na,K-ATPase β subunits, as described in MATERIALS AND METHODS, metabolically labeled for 24 h and subjected to a 48-h chase period before preparation of digitonin extracts and immunoprecipitation with an α antibody under denaturing (A and B) or nondenaturing (C) conditions. Shown are fluorograms of immunoprecipitates resolved by SDS-PAGE. (A) Truncated α-proteins containing the 2 N-terminal membrane pairs (lanes 1–6) and, in addition, the large cytosolic loop (lanes 7 and 8) of Na,K-ATPase α subunits are not degraded during a 48-h chase period, but a truncated α-protein containing transmembrane segment M5 (lanes 9 and 10) or M6 (lanes 11 and 12) is degraded. (B) M1–7 up to M1–9 truncated α-proteins are degraded (lanes 1–8) during the chase period similar to full-length (fl), wild-type α subunits (lanes 9 and 10). (C) Association of β subunits with truncated α-proteins. Indicated are the positions of truncated α-proteins, coimmunoprecipitated β subunits, and proteins of known molecular masses. The weak band visible in lanes 3 and 4 and migrating at the level of the fully glycosylated β subunit seen in lane 10 represents an artifact occasionally produced at the migration front of the antibodies (Jaunin et al., 1993). cg, core glycosylated β subunits; fg, fully glycosylated β subunits. One of three or four representative experiments is shown. Also summarized at right are results obtained by Béguin et al. (1998) on the putative membrane topology of truncated α-proteins determined by an RGS assay. Indicated is the percentage of truncated α-proteins containing the ectodomain of a β subunit that became glycosylated in an RGS assay performed in Xenopus oocytes in the absence or presence of β subunits. The loops M5/M6 and M7/M8 are only partially inserted in the membrane until complete synthesis and β association have occurred. For further description, see RESULTS. el, extracellular loop between M7 and M8 containing a β-assembly domain. The cytoplasmic loop is drawn as a gray line, indicating that it is not on the scale.