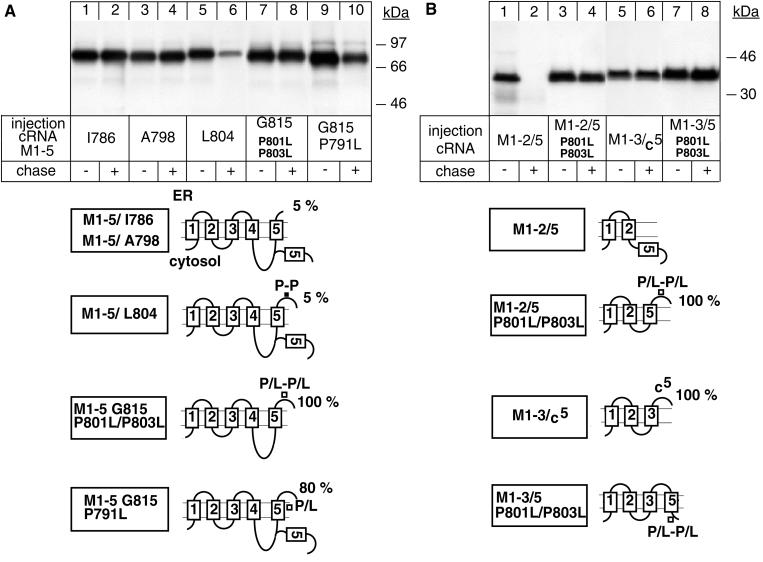
Figure 2.
The extracytoplasmic loop between M5 and M6 contains a putative P-P degradation motif. Oocytes were injected with cRNA coding for truncated α-proteins, treated as described in Figure 1, and digitonin extracts were immunoprecipitated with an α antibody under denaturing conditions. (A) P801L/P803L mutations favor membrane insertion of M5 and impede degradation of an M1–5 G815 α-protein. (B) Transposition of M5 mediates degradation of the intrinsically stable M1–2 α-protein from the cytoplasmic side. One of two to four representative experiments is shown. At the bottom, putative topology models determined by an RGS assay are shown, indicating the percentage of glycosylated ER forms of truncated α-proteins. Also indicated are the positions of the mutated proline residues.