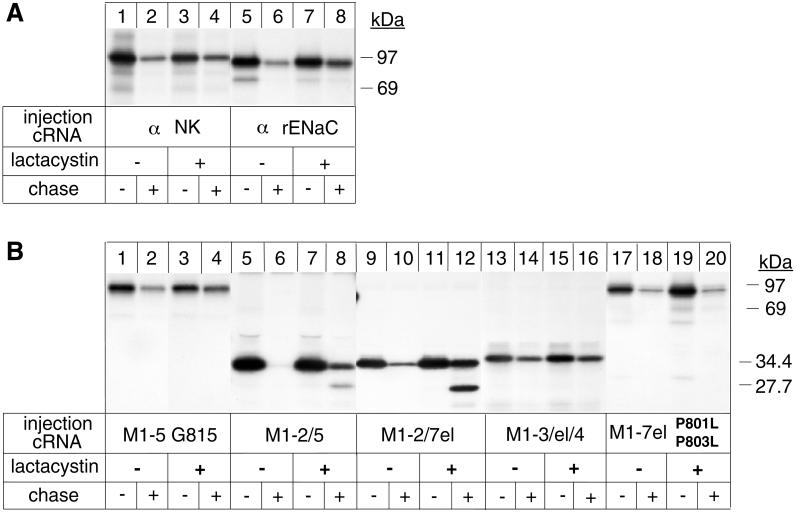
Figure 5.
Proteasome-dependent and -independent degradation of Na,K-ATPase α variants. Oocytes were preincubated or not with 50 μM lactacystin for 16 h before injection with cRNA coding for Na,K-ATPase α subunits (α NK), for truncated α variants, or for α subunits of the renal, epithelial Na channel (α rENaC). After a 6-h pulse with [35S]methionine in the absence or presence of 100 μM lactacystin and a 24-h chase in the absence or presence of 25 μM lactacystin, digitonin extracts were prepared, and immunoprecipitations under denaturing conditions were performed with an Na,K-ATPase α antibody (A, lanes 1–4, and B) or with an α rENaC antibody (A, lanes 5–8). (A) Degradation of unassembled Na,K-ATPase α subunits is partially inhibited by the proteasomal inhibitor lactacystin, similar to that of α rENaC. (B) Partial inhibition of degradation by lactacystin of topology-representative α variants. Indicated is the molecular mass of the uncleaved M1–2/7el α-protein and that of a proteolytic fragment accumulating in the presence of lactacystin. One of two or three representative experiments is shown.