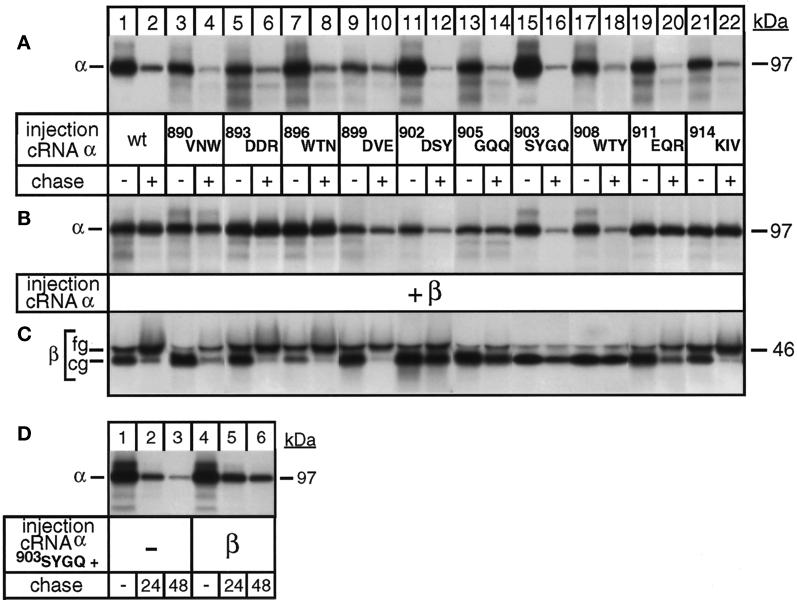
Figure 6.
The SYGQ motif in the M7/M8 extracellular loop of Na,K-ATPase α subunits is necessary but not sufficient for the correct folding of Na,K-ATPase α subunits after β assembly. Oocytes were injected with α cRNA coding for full-length α subunits with alanine replacements of the amino acids indicated in the absence (A) or presence (B–D) of cRNA coding for β subunits and subjected to a 24-h pulse and a 48-h chase period (A–C) or a 6-h pulse and 24- and 48-h chase periods (D) before preparation of digitonin extracts and immunoprecipitation with an α antibody (A, B, and D) or a β antibody (C) under denaturing conditions. (A) Alanine variants expressed in the absence of β subunits are degraded during a 48-h chase period (lanes 3–22) similar to wild-type (wt) α subunits (lanes 1 and 2). (B) A region encompassing D902 up to Y910 is essential for β interaction and stabilization. (C) β subunits coexpressed with alanine variants affected in the region encompassing D902 up to Y910 remain in their core glycosylated (cg) ER form (lanes 11–18), indicating that they cannot efficiently associate with α variants, in agreement with the lack of α stabilization. β subunits coexpressed with alanine variants affected in the region V890 up to E901 or E911 up to V916 become fully glycosylated (fg), indicating that the α-β complexes have left the ER. One of three representative experiments is shown. (D) Partial stabilization of 893SYGQ alanine variants by β subunits. One of two representative experiments is shown.