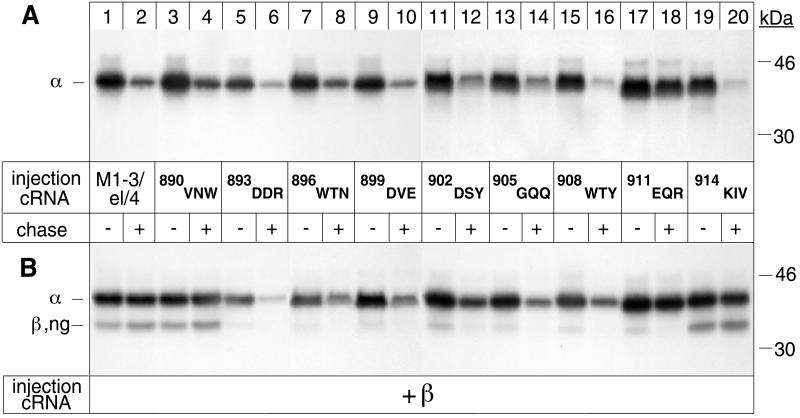
Figure 7.
The β assembly domain contains a putative degradation signal. Oocytes were injected with α cRNA coding for M1–3/el/4 α-proteins (lanes 1 and 2) or M1–3/el/4 α variants with alanine replacements of the amino acids indicated in the absence (A) or presence (B) of cRNA coding for β subunits and subjected to a 24-h pulse and a 48-h chase period before preparation of digitonin extracts and immunoprecipitation with an α antibody. (A) Alanine replacement of 901EQR impedes degradation of M1–3/el/4 α-proteins. (B) β assembly with M1–3/el/4 α-proteins depends on the integrity of a region encompassing D893 up to R913. Immunoprecipitated samples shown in B were treated with Endo H to permit separation of the α and β bands. One of two or three representative experiments is shown.