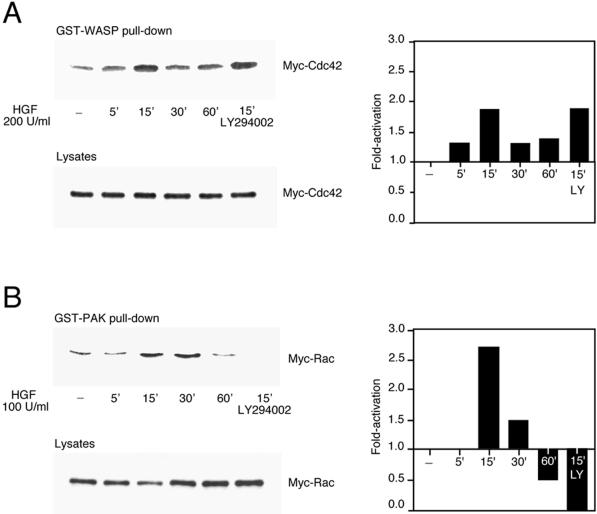
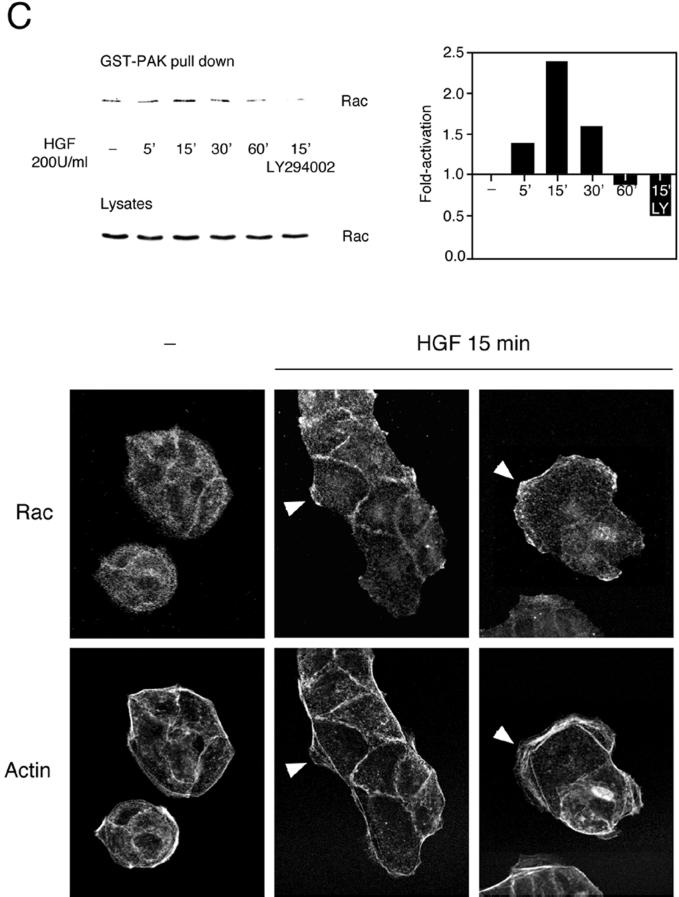
Figure 2.
HGF-induced Cdc42 and Rac activation in MDCK cells. MDCK cells transiently expressing Myc-tagged G12Cdc42 (A) or G12Rac (B), or MDCK cells expressing endogenous Rac (C), were stimulated with 100 or 200 U/ml HGF for the indicated times. Cells were lysed, and proteins in equal amounts were incubated with GST-WASP (aa 201–321) (A) or GST-PAK (aa 56–272) (B and C) proteins and purified on glutathione–Sepharose beads. Beads were washed, and bound proteins were separated on a 15% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The levels of Myc-tagged Cdc42 (A), Myc-tagged Rac (B), and endogenous Rac (C) pulled down by the fusion proteins, as well as the levels present in whole cell lysates, were evaluated by Western blotting with the 9E10 Myc (A and B) or Rac (C) mAbs. The corresponding graphs indicate the activation detected over basal levels present in unstimulated cells. (C, bottom) HGF-induced translocation of Rac in MDCK cells. MDCK cells stimulated with HGF (5 U/ml) for 15 min were fixed in 3.7% formaldehyde in PBS, permeabilized in Triton X-100 (0.2%), and stained with anti-Rac, followed by incubation with anti-mouse Alexa 488 together with TRITC-phalloidin. Fluorescent images were obtained by laser confocal microscopy. Note the presence of Rac in the membrane ruffles found at the edges of lamellipodia (arrowheads).