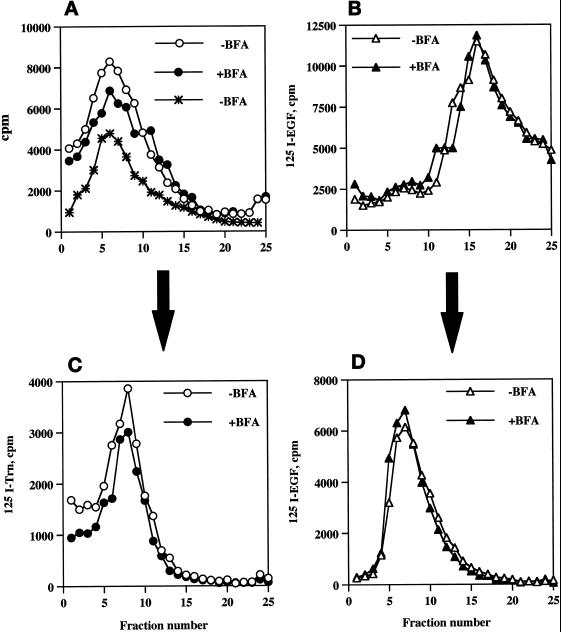
Figure 6.
Compartmentalization of internalized 125I-Trn and 125I-EGF in the presence or absence of BFA. PC12 cells expressing wild-type ssHRPP-selectin were fed with 125I-Trn for 1 h at 37°C in the presence (●) or absence (○) of 10 μg/ml BFA added in the medium during last 30 min of incubation. Parallel dishes were incubated with 125I-EGF for 1 h on ice in the presence or absence of BFA for the last 10 min of incubation, washed, and transferred to 37°C for 20 min to label late endosomes in the presence (▴) or absence (▵) of BFA. One dish labeled with 125I-EGF for 1 h on ice was washed and allowed to internalize ligand for 2 min at 37°C with no BFA added (A, ∗). After removal of the noninternalized ligand, cells were rinsed with HB and homogenized, and a PNS was centrifuged on the 1–16% Ficoll velocity gradients (A and B). After fractionation, the peak containing 125I-Trn (fractions 5–10; A) was collected and then recentrifuged on a 3–16% Ficoll gradient (C), whereas the peak containing 125I-EGF (fractions 13–19; B) was collected and recentrifuged on a 0.9–1.85 M sucrose equilibrium gradient (D) as described in MATERIALS AND METHODS.