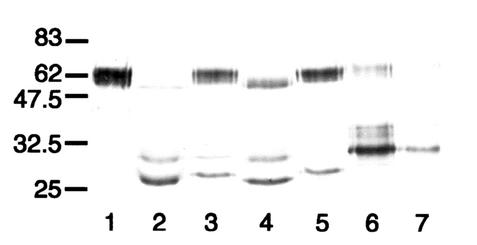
FIG. 7.
Western blot analysis of the action of streptococcal IgA1 proteases on mutant antibody T228/236V. After separation under reducing conditions, digests were probed with an anti-human IgA α chain-specific peroxidase conjugate that bound to epitopes in the Fab region. T228/236V was untreated (lane 1) or treated with the IgA1 proteases of S. pneumoniae SK690 (lane 2), S. sanguis SK4 (lane 3), S. oralis SK10 (lane 4), S. mitis SK564 (lane 5), and the type 2 IgA1 protease of N. meningitidis HF13 (lane 6). Wild-type recombinant IgA1 treated with the latter enzyme is shown in lane 7, and the positions of molecular mass markers (in kilodaltons) are indicated on the left. Mutant antibody T228/236V treated with streptococcal IgA1 proteases was cleaved to give Fab fragments with masses consistent with cleavage at or near the peptide bond between residues 227 and 228, as in wild-type IgA1, and also, for most of them, at or near the bond between residues 235 and 236.